MOS Type Gas Sensor Principle
In clean air, donor electrons in tin dioxide are attracted toward oxygen which is adsorbed on the surface of the sensing material, preventing electric current flow.
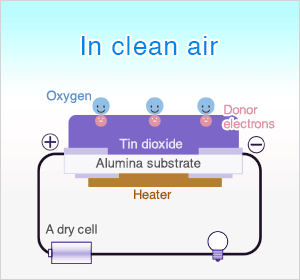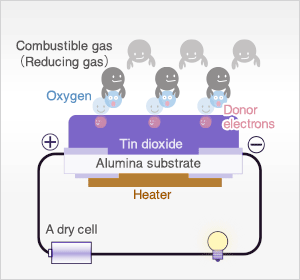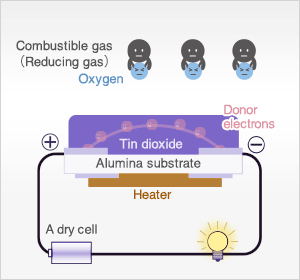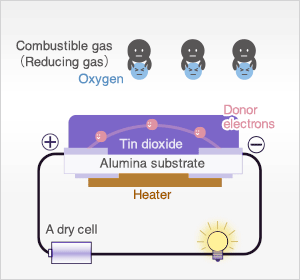
Image Credits : figaro
In the presence of reducing gases, the surface density of adsorbed oxygen decreases as it reacts with the reducing gases. Electrons are then released into the tin dioxide, allowing current to flow freely through the sensor. The current flow will give the equivalent readings of the measured gas.
Also Read : Gas Detectors Standards
Principle :
In the most extreme case where oxygen concentration is 0%, when metal oxide sensor material (typically tin dioxide [SnO2-x]) is heated at high temperature such as 400˚C, free electrons flow through the conjoined parts (grain boundary) of tin dioxide crystals.
In clean air (approx.. 21% O2), oxygen is adsorbed on the metal oxide surface. With its high electron affinity, adsorbed oxygen attracts free electrons inside the metal oxide, forming a potential barrier (eVs in air) at the grain boundaries.
This potential barrier prevents electron flow, causing high sensor resistance in clean air.
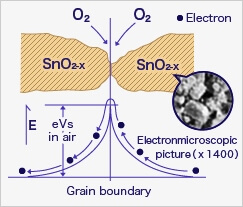
When the sensor is exposed to combustible gas or reducing gas (such as carbon monoxide), the oxidation reaction of such gas with adsorbed oxygen occurs at the surface of tin dioxide.
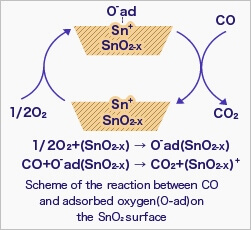
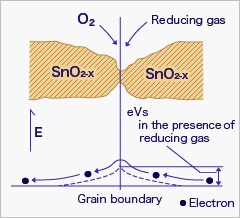
As a result, the density of adsorbed oxygen on the tin dioxide surface decreases, and the height of the potential barrier is reduced. Electrons easily flow through the potential barrier of reduced height, and the sensor resistance decreases.
Gas concentration in air can be detected by measuring the resistance change of MOS-type gas sensors. The chemical reaction of gases and adsorbed oxygen on the tin dioxide surface varies depending on the reactivity of sensing materials and working temperature of the sensor.
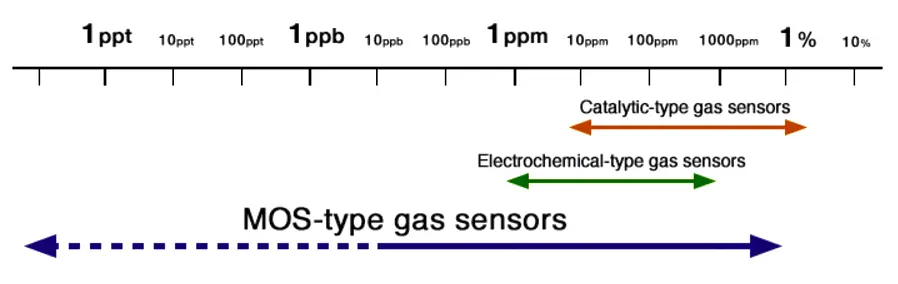
MOS Gas Sensors :
Also Read : Fire Suppression System Principle
Credits : figaro