Total Organic Carbon (TOC) measurement is commonly used to determine the degree of organic contamination in water.
Total Organic Carbon (TOC) is an indirect measure of organic molecules present in water and measured as carbon. Organic molecules are introduced into the water from the source water, from purification, and from distribution system materials. TOC is measured for both process control purposes and to satisfy regulatory requirements.
Analytical technologies utilized to measure TOC share the objective of completely oxidizing the organic molecules in an aliquot of sample water to carbon dioxide (CO2), measuring the resultant CO2 concentration, and expressing this response as carbon concentration. All technologies must discriminate between the inorganic carbon, which may be present in the water from sources such as dissolved CO2 and bicarbonate, and the CO2 generated from the oxidation of organic molecules in the sample.
One approach used to measure TOC involves subtracting the measured inorganic carbon (IC) from the measured total carbon (TC), which is the sum of organic carbon and inorganic carbon:
TOC = TC – IC.
TOC Technologies
The main difference between TOC technologies is how they oxidize organics.
All Total Organic Carbon (TOC) analyzers perform two functions: oxidizing organic carbon in water to CO2 and measuring the CO2 produced. What makes each TOC analyzer different is the method it uses to oxidize the organics in the water sample and the methods used to detect the resulting CO2.
Main Detection Methods
The three main methods of CO2 detection used commercially are:
- Non-dispersive infrared (NDIR)
- Direct Conductometric (Non-selective Conductometric)
- Membrane Conductometric Detection (Selective Conductometric)
NDIR TOC detectors measure CO2 in the gaseous phase, while conductometric TOC detectors measure CO2 in the liquid phase.
Non-Dispersive InfraRed (NDIR)
The non-dispersive infrared (NDIR) detector takes a preliminary reading to determine a baseline. When the sample enters the NDIR cell, the carbon dioxide molecules absorb the infrared light coming from the source, reducing the total transmittance of infrared light that reaches the detector. After all carbon dioxide has been removed from the cell, the percent transmittance returns to 100%.
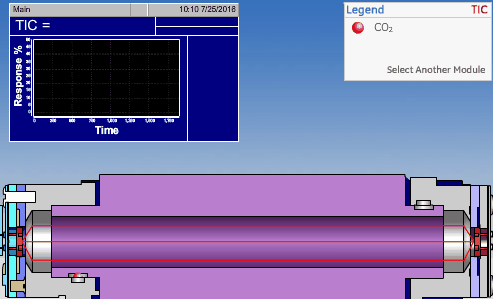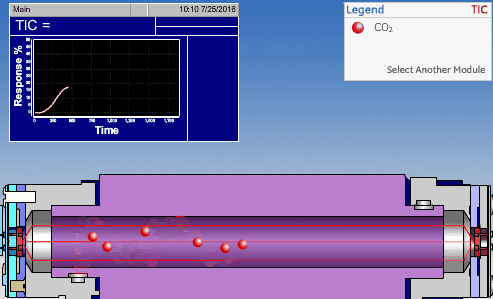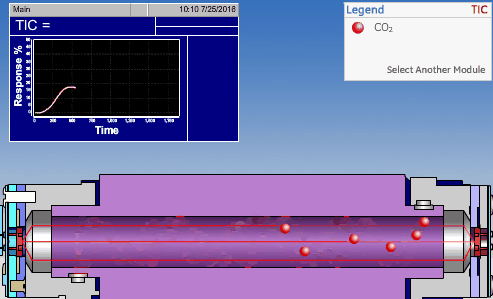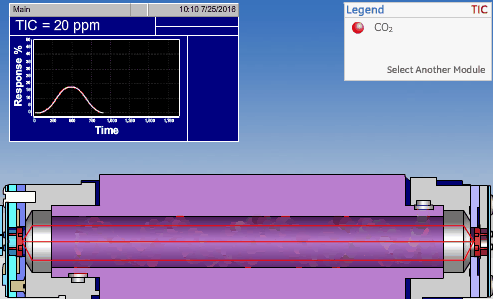
Conductometric Technologies
Conductometric TOC detectors measure CO2 in the liquid phase. The two conductometric type detectors are Direct Conductometric and Membrane (or Selective) Conductometric. The two conductometric-type detectors have stable calibration and high sensitivity. The primary difference between the two conductometric types is that the direct detector is susceptible to interference from ionic contamination, acids, bases, and halogenated organics.
In the membrane-based conductometric method, the membrane is a protective barrier to interfering ions, enabling the analysis of CO2 only. The result is a more accurate TOC reading.
i like this site so match, it’s a very good information about all instrumentation
thank you my friend for your information
i need some help about some problems about CO2 analyzer
if you can help me with pleasure