The measurement methods of the oxygen analyzers currently available in the industry can be classified into the following categories.
- Zirconia type measurement system
- Paramagnetic type
- Optical type
- Electrochemical type
Since each of the measurement methods has its advantages and disadvantages, it is important to select an oxygen analyzer of an appropriate method for your application and usage.
The following describes an overview of each of the measurement methods and their advantages and disadvantages.
(1) Zirconia type measurement system : Concentration cell system
A solid electrolyte like zirconia exhibits conductivity of oxygen ions at high temperature.
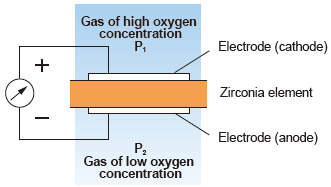
As shown in the figure, when porous platinum electrodes are attached to both sides of the zirconia element to be heated up and gases of different partial oxygen concentrations are brought into contact with the respective surfaces of the zirconia, the device acts as an oxygen concentration cell. This phenomenon causes an electromotive force to be generated between both electrodes according to Nernst’s equation. And it is proportional oxygen concentration.
| Advantages : | Can be directly installed in a combustion process such as a boiler’s flue and requires no sampling system, and response is faster. |
| Disadvantages : | If the sample gas contains a flammable gas, a measurement error occurs (combustion exhaust gas causes almost no problem because it is completely burned). |
(2) Zirconia type measurement system : Limiting Current type
As shown in the figure below, if the flow of oxygen into the cathode of a zirconia element heated to high temperature is limited, there appears a region where the current becomes constant even when the applied voltage is increased.This limited current is proportional to the oxygen concentration.
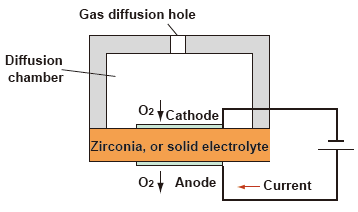
| Advantages : | • • |
Capable of measuring trace oxygen concentration. Calibration is required only on the span side (air). |
| Disadvantages : | • | If the sample gas contains a flammable gas, a measurement error occurs. |
| • | The presence of dust causes clogging of the gas diffusion holes on the cathode side; a filter must be installed in a preceding stage. |
(3) Magnetic type measurement system : Paramagnetic system
This is one of the methods utilizing the paramagnetic property of oxygen. When a sample gas contains oxygen, the oxygen is drawn into the magnetic field, thereby decreasing the flow rate of auxiliary gas in stream B. The difference in flow rates of the two streams, A and B, which is caused by the effect of flow restriction in stream B, is proportional to the oxygen concentration of the sample gas. The flow rates are determined by the thermistors and converted into electrical signals, the difference of which is computed as an oxygen signal.
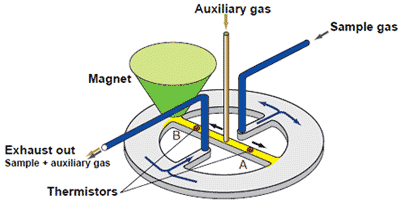
| Advantages : | • | Capable of measuring flammable gas mixtures that cannot be measured by a zirconia oxygen analyzer. |
| • | Because there is no sensor in the detecting section in contact with the sample gas, the paramagnetic system can also measure corrosive gases. | |
| • | Among the magnetic types, the paramagnetic system offers a faster response time than other systems. | |
| • | Among the magnetic types, the paramagnetic system is more resistant to vibration or shock than other systems. | |
| Disadvantages : | Requires a sampling unit corresponding to the sample gas properties or applications. |
(4) Optical type : Tunable Diode Laser measurement system
Tunable Diode Laser (or TDL) measurements are based on absorption spectroscopy. The TruePeak Analyzer is a TDL system and operates by measuring the amount of laser light that is absorbed (lost) as it travels through the gas being measured. In the simplest form a TDL analyzer consists of a laser that produces infrared light, optical lenses to focus the laser light through the gas to be measured and then on to a detector, the detector, and electronics that control the laser and translate the detector signal into a signal representing the gas concentration.
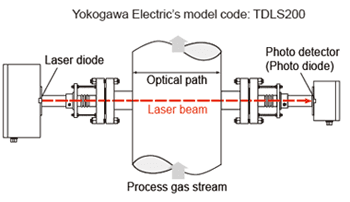
Gas molecules absorb light at specific colors, called absorption lines. This absorption follows Beers law.
TDL Analyzers are effectively infrared analyzers which obey the Beer-Lambert Law.
I = Io ·e-E ·G ·L
where I is the radiation intensity after absorption,
I0 is the initial radiation intensity,
E is the extinction coefficient,
G is the gas concentration,
and L is the path length of the measurement area.
| Advantages : | • | Capable of measuring a number of near infrared absorbing gases in difficult process applications. |
| • | Capability of measuring at very high temperature, high pressures and under difficult conditions (corrosive, aggressive, high particulate service). | |
| • | Most applications are measured in-situ, reducing installation and maintenance costs. | |
| Disadvantages : | The installation of the flange is necessary for both sides of the process. |
(5) Electrochemical type : Galvanic cell type
If oxygen is dissolved via the diaphragm in an electrolytic solution in which an anode (base metal) and cathode (noble metal) are adjacent to each other, a current proportional to the quantity of dissolved oxygen is generated. The amount of oxygen passing through the diaphragm is proportional to the partial oxygen pressure of the sample gas, therefore, the oxygen concentration can be determined by measuring the current.
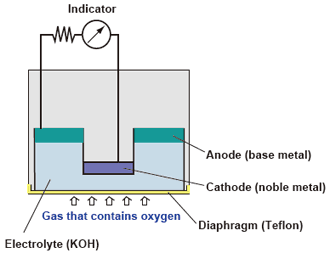
| Advantages : | • | The detecting system can be made compact; this measurement system is available in portable or transportable form. |
| • | Relatively inexpensive in comparison with oxygen analyzers of other measurement systems. | |
| Disadvantages : | The cell life is limited. As it is a kind of oxygen cell, the galvanic cell deteriorates even if not used. In general, it should be replaced approximately every year. |
Application examples for each oxygen analyzer
Oxygen concentrations are measured for a variety of purposes, such as energy conservation, air pollution prevention, safety management, and quality control.
The following lists major application examples by measurement method.
| Concentration cell system Zirconia Oxygen Analyzer | |
| Package boiler combustion control, gas fired Combustion control of power generation boilers, gas fired Combustion control of pulverized coal boilers Combustion control of hot stoves for steelmaking Heating and combustion exhaust gas control of coke ovens for steelmaking Low-oxygen concentration control of reheating and soaking furnaces for steelmaking Air leakage detection of sintering furnaces for steelmaking Lime kiln combustion control Cement kiln combustion control Combustion control of heating furnaces for oil refinery & petrochemical industry Naphtha cracking furnaces Incinerator combustion control Oxygen concentration measurement in oxygen enrichment facilities Oxygen concentration measurement of exhaust gas from activated sludge process equipment |
|
| Limiting Current type Zirconia Oxygen Analyzer | |
| Oxygen concentration control of N2 reflow furnaces Atmospheric control of semiconductor manufacturing equipment N2 and air purity control for air separators Oxygen deficiency prevention Oxygen concentration control of glove boxes for research and development and parts machining Oxygen concentration control of experimental clean rooms for environment, fermentation, biochemistry, etc. Continuous measurement of flow gases during food packaging |
|
| Paramagnetic system Oxygen Analyzer | |
| Low-oxygen concentration control of CDQ plants for steelmaking Oxygen concentration control of gas containing a flammable gas Safety control (explosion prevention) at various plants Measurement of trace oxygen concentration in various manufacturing processes City gas quality control |
|
| Galvanic cell system Oxygen Analyzer | |
| Oxygen deficiency prevention Package boiler combustion control, gas fired |
|
| Tunable Diode Laser Analyzer(TDLS) | |
| Combustion control of heating furnaces Incinerator combustion control Process control |
|
Also Read: pH Analyzer Interview Questions & Answers
Please give me material is all gas detection methods.to my mail I’d.
I need a trace oxygen online analyser which is installed in plant. Detail catalog is required.