In this article, we will discuss the Infrared Flue Gas analyzer and its working principle.
Here the portion of the infrared spectrum gets absorbed when the infrared beam passes through certain gases like carbon monoxide and carbon dioxide.
The amount of gas that is passed can be determined by the amount of a particular frequency of light absorbed by the gas when the light is passed through the gas.
The gases like carbon monoxide, carbon dioxide, and many other gases have infrared absorption characteristics. But elemental diatomic gases such as oxygen, hydrogen, nitrogen, etc. do not exhibit such characteristics.
The concentration of any of the components that indicates the infrared absorption characteristics in a mixture is determined by its unique absorption pattern.
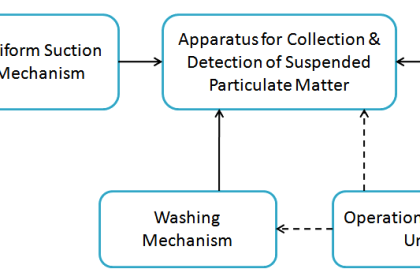
Infrared Flue Gas Analyser
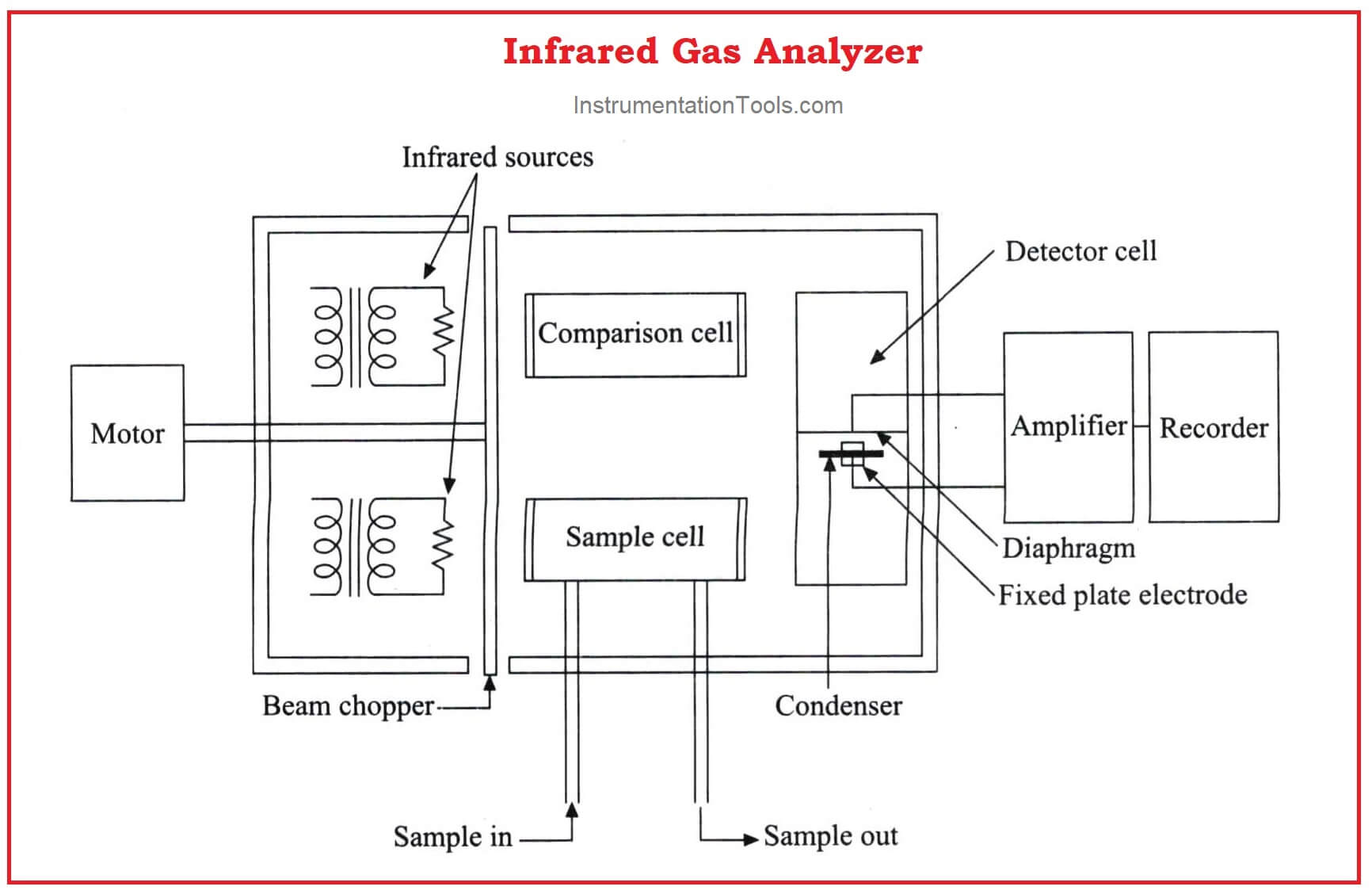
The above figure shows an infrared analyzer using infrared techniques. Two similar infrared sources may be Nichrome filaments used as infrared radiation sources.
The light beams from the sources are made to travel through the sample cell and comparison cell respectively.
A mechanism called beam chopper in the analyzer in between the source and the cells is operated so that it alternatively interrupts the light radiation to the sample cell and comparison cell.
The emergent radiation from both the sample cell and the comparison cell enters the opposite ends of a detector cell.
This detector cell is divided into two compartments by a metallic diaphragm and is filled with the gas to be measured at the same pressure.
Further processing is made with the condenser connected with the amplifier and is formed by combining a metallic diaphragm and the fixed electrode.
The different molecules present in the air absorb different light frequencies. The absorbed frequency which is measured will clearly say the relation to the amount of particular gas in the air.
For every optical analyzer and for every absorption type, the absorption spectrum of infrared radiation is unique for various gases.
The beer Lambert’s law gives the fundamental equation relating photon absorption to substance concentration.
Analyzer Working Principle
The comparison cell is filled with any of the diatomic gases like O2, N2, or Hydrogen. That does not absorb infrared energy.
The sample cell is also filled with the same diatomic gas as in the comparison cell for adjusting the zero of the analyzer. Then the sample gas is made to pass through the sample cell.
The absorption rate of infrared energy depends upon the components present in the sample.
In this case, Let us assume measuring CO components.
The detector cell is filled with Carbon Monoxide gas on both sides. When the unit is under operation the energy received from the sample side in the detector will be less by an amount equivalent to the CO2 component present in the sample compared to the energy received from the comparison cell.
The difference in energy levels received and finally, absorbed by CO2 present on both sides of the detector cell will cause temperature and hence pressure difference.
As the chopper allows the entry only alternatively, the metal diaphragm vibrates at the chopping frequency (usually varies from 2 Hz to 10 Hz) with the magnitude of the displacement from the fixed electrode equivalent to CO2 content with the sample.
The measure of CO2 value determines the capacitance of the condenser.
The condenser capacitance is measured using an amplifier and calibrated for indicating and recording. If the same analyzer is to be used for measuring CO, the detector cell will be filled with CO, and the calibration is done accordingly.
An improved version with a single infrared source is shown in the figure below.
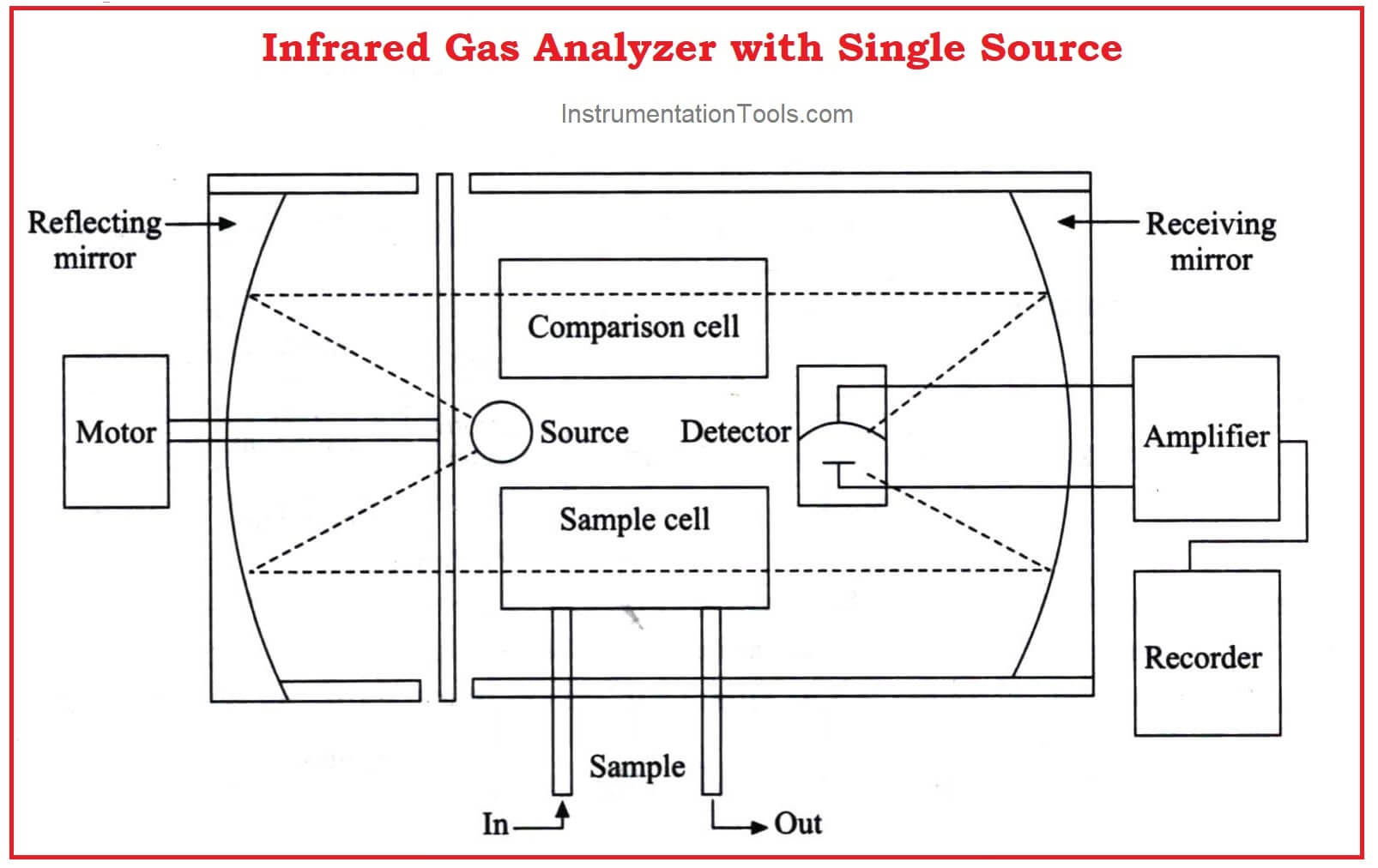
Similar sources are eliminated which may cause minor differences.
Infrared analyzers can be used to analyze independently the CO, CO2, NOx, SOx, etc. that are present in the flue gas of a thermal power plant.