One of the manual chromatography methods taught to beginning chemists is thin-layer chromatography, also known as TLC.
An illustrated sequence showing thin-layer chromatography appears here:
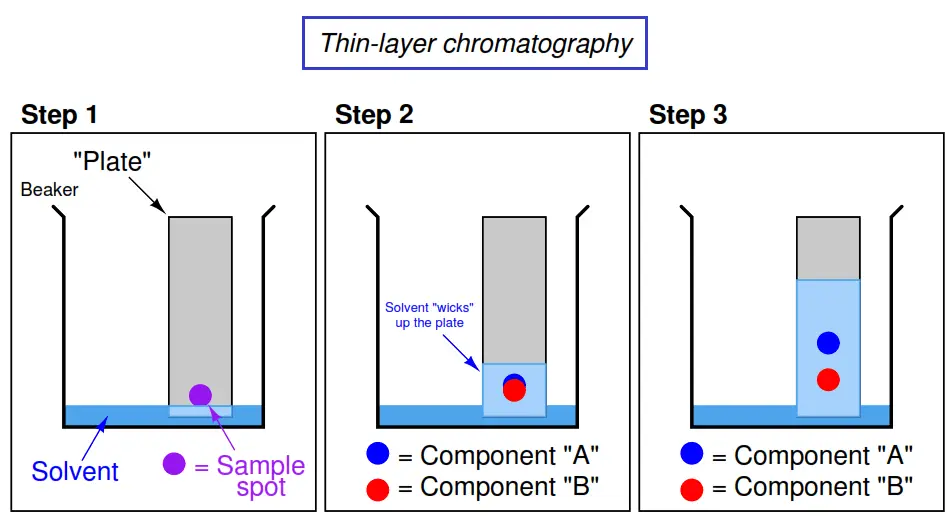
As solvent wicks up the surface of the plate, it carries along with it all components of the sample spot. Each component travels at a different speed, separating the components along the plate over time.
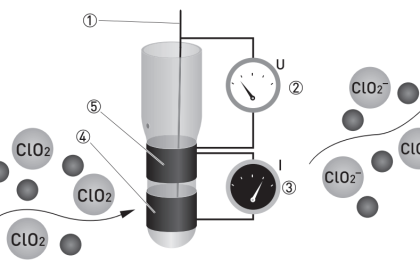
The simplest forms of chromatography reveal the chemical composition of the analyzed mixture as residue retained by the stationary phase. In the case of thin-layer chromatography, the different liquid compounds of the mobile phase remain embedded in the stationary phase at distinct locations after sufficient “developing” time. The same is true in paper-strip chromatography where a simple strip of filter paper serves as the stationary phase through which the mobile phase (liquid sample and solvent) travels: the different species of the sample remain in the paper as residue, their relative positions along the paper’s length indicating their extent of travel during the test period. If the chemical species happen to have different colors, the result will be a stratified pattern of colors on the paper strip (Note).
Note : This effect is particularly striking when paper-strip chromatography is used to analyze the composition of ink. It is really quite amazing to see how many different colors are contained in plain “black” ink!


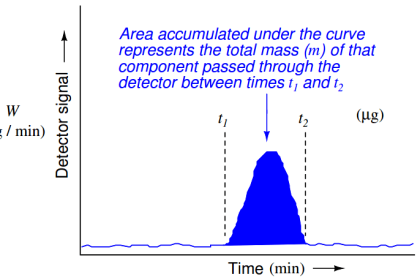





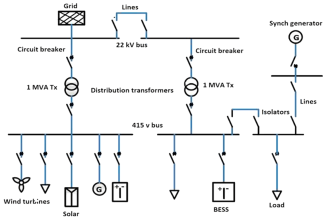
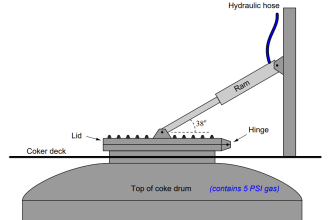

NEED REFERENCE
FROM WHICH BOOK THIS TOPIC IS TAKEN