What is dissolved oxygen ?
Dissolved oxygen refers to oxygen dissolved in water. Its concentration is expressed as the amount of oxygen per unit volume and the unit is mg/L. Biologically, oxygen is an essential element for respiration of underwater life and also acts as a chemical oxidizer. The solubility of oxygen in water is affected by water temperature, salinity, barometric pressure, etc. and decreases as water temperature rises.
Measurement of dissolved oxygen by the membrane electrode method
The membrane electrode method measures a diffusion current or reduction current generated by the concentration of dissolved oxygen or partial pressure of oxygen to obtain the concentration of dissolved oxygen. This method is not affected by the pH value of water being measured, oxidation and reduction substances, color, turbidity, etc. and the measurement method offers good reproducibility.
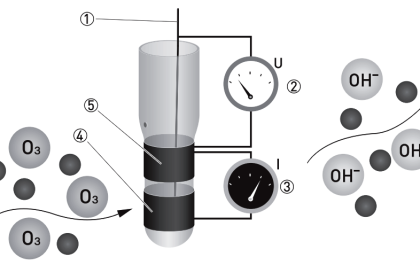
When a sensor is inserted into water, an air layer forms on the membrane (Teflon membrane). The oxygen partial pressure (concentration) in the air layer is in equilibrium with the concentration of dissolved oxygen in the water. The membrane electrode method measures the oxygen concentration in the gas phase to indirectly obtain the concentration of dissolved oxygen in water.
There are two types of membrane electrode method:
Galvanic cell method, and
Polarographic method.
These methods differ only in the presence or absence of an external applied voltage and have the same performance, features, and usage method.
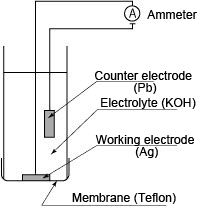
(1) Galvanic cell method
The membrane has high permeability to oxygen and is constructed so that the electrodes and electrolyte are isolated from the water being measured. The counter electrode is a base metal and the working electrode is a noble metal and potassium hydroxide is used as the electrolyte. Oxygen passes through the membrane and is reduced on the working electrode, and so the method measures the reduction current flowing between both electrodes, which is proportional to the concentration of dissolved oxygen.
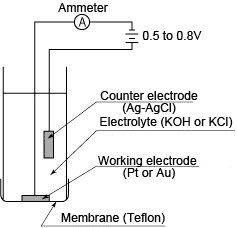
(2) Polarographic method
The sensor construction is almost the same as that of the galvanic cell method. The counter electrode is silver-silver chloride and the working electrode is gold or platinum. When a voltage of 0.5–0.8 V is applied between both electrodes, oxygen that has permeated through the membrane initiates a reduction reaction on the working electrode, causing a polarographic limiting current to flow which is proportional to the oxygen concentration. This method measures the concentration of dissolved oxygen based on this current value.
Calibration of the dissolved oxygen analyzer
Calibration of the dissolved oxygen analyzer is performed in the following situations:
| – | When a new dissolved oxygen sensor is installed |
| – | When the membrane assembly is replaced and/or the electrolyte solution is replaced |
| – | When the sensor has been disassembled and reassembled for maintenance |
| – | When a measuring error after cleaning exceeds the acceptable deviation from the reference method |
There are the following methods for calibrating the dissolved oxygen analyzer:
| Air calibration: | |
| Performs span calibration in ambient air. This is the most common and easiest way to calibrate the analyzing system, and the method is generally satisfactory. The calibration table lists experimental values at a relative humidity of 70%. It is important to wash any contamination from the sensor membrane, and then wipe off remaining water droplets from the membrane gently with soft paper, etc. | |
| Span calibration in air-saturated water: | |
| This method complies with ISO 5814; perform this calibration if you wish to calibrate the analyzer more accurately. | |
| Zero calibration in sodium sulfite solution: | |
| This calibration takes much time, and is not generally required. Real solution calibration: Measurement is made using an instrument based on the water being measured and calibration is performed in accordance with the value obtained. |
|
Also Read: Conductivity Analyzer Interview Questions & Answers