The number of impurities present in water and steam can be found out approximately by carrying the following measurements.
Conductivity Meters
- Electrolytic conductivity is the capacity of a solution to pass an electric current. Conductivity measurements are done for determining the salt content present in the feed water, condensate, and steam.
- The number of impurities present in water improves conductivity, which means the rate of impurity and conductivity are in direct proportion to each other.
- The electrical conductivity of pure water is minimum.
- Conductivity measurement helps to determine the amount of total dissolved solids present in water.
- The conductivity of a solution is measured in micromhos per centimeter or micro-siemens per centimeter at 25°C,
- The acceptable limit is approximately 0.3 μ-mhos/cm³.
- Conductivity is the reciprocal of resistivity of a centimeter cube of water between two plates 1 cm² in the area and placed 1 cm apart.
- Conductivity increases with increasing impurities present in the solution.
- The conductivity cell is used to measure conductivity.
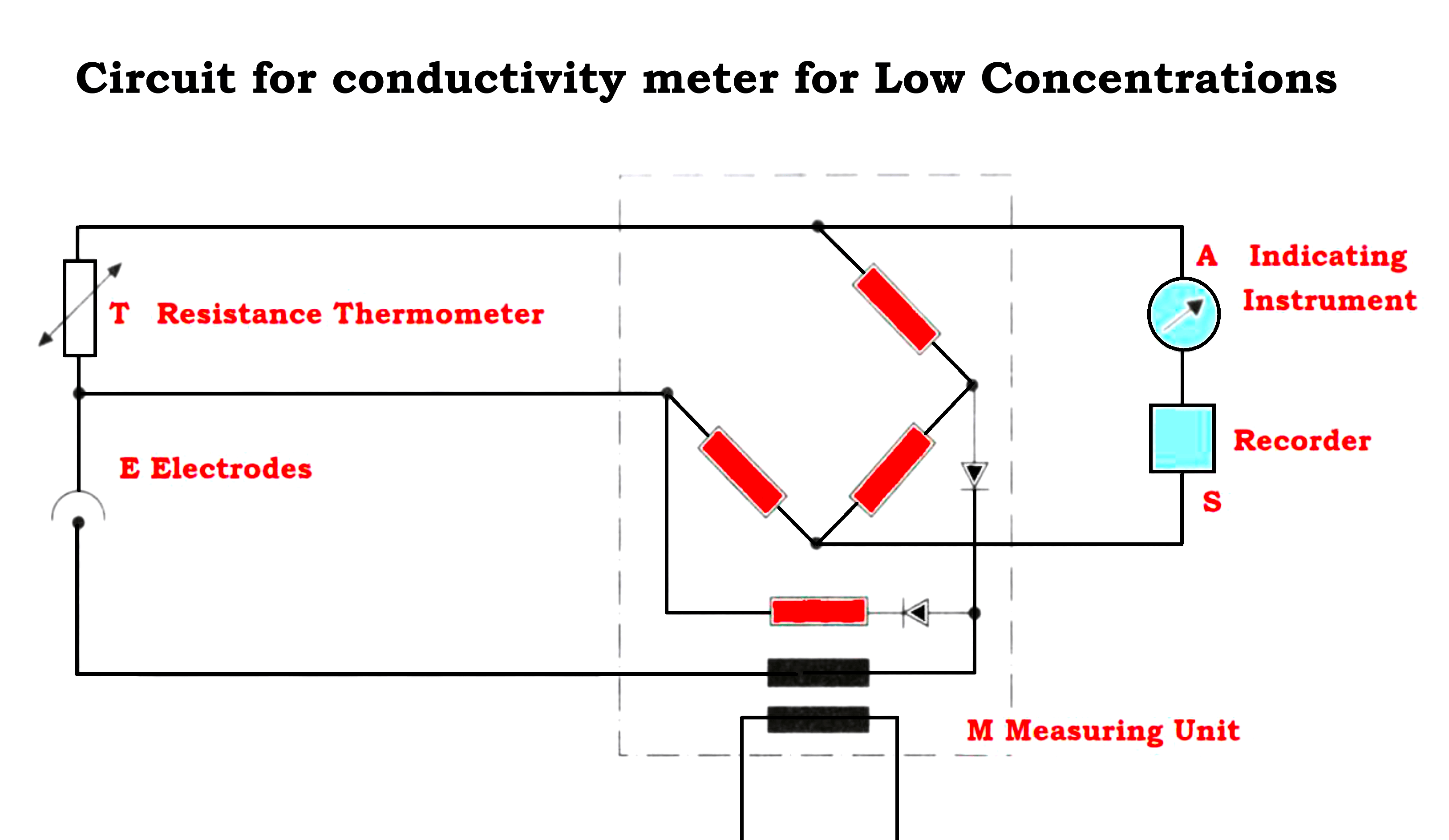
- The relationship for resistance, conductivity, and cell constant is shown below
Resistance = (Resistivity x Length of Sample) / (The cross-sectional area of plates)
Resistance = ρ*L/a
And
Conductivity= 1/ Resistivity = 1/ρ = (L/Ra) mho/cm = K
Where
R = Cell resistance in ohms.
ρ= Resistivity in ohms cm²/cm
L= Sample length in cm
a = Area in cm²
K= Conductivity in mhos – cm/cm²
L/a = Cell constant in 1/cm = C
The term C = is a cell constant for a given set of electrode dimensions.
In contrast to pH measurement, the value depends on the hydrogen ion concentration alone.
For a given sample, conductivity is defined as a function of the concentration of all ions present in it.
Typical ranges of conductivity meters are listed below.
| Level | Cell constant C | Range’s mhos/cm |
| Very low concentration | 0.006 | 0-0.6 0-4 |
| Low concentration | 0.06 | 0-10 0-200 |
| Medium concentration | 1 | 200-1000 1000-5000 |
- For one particular kind of salt, conductivity is a measure of the salt content. Generally, in average cases, the composition of the feed water is constant hence the sufficient correct result is obtained.
- As the conductivity meter is principally used for monitoring the salt content it is calibrated in mg- Nacl per liter instead of micromhos per centimeter. (1 mg Nacl/liter = 1.9 µmhos/cm at 26°C)
- The accuracy of conductivity measurements depends to a considerable extent on the shape and arrangement of electrodes.
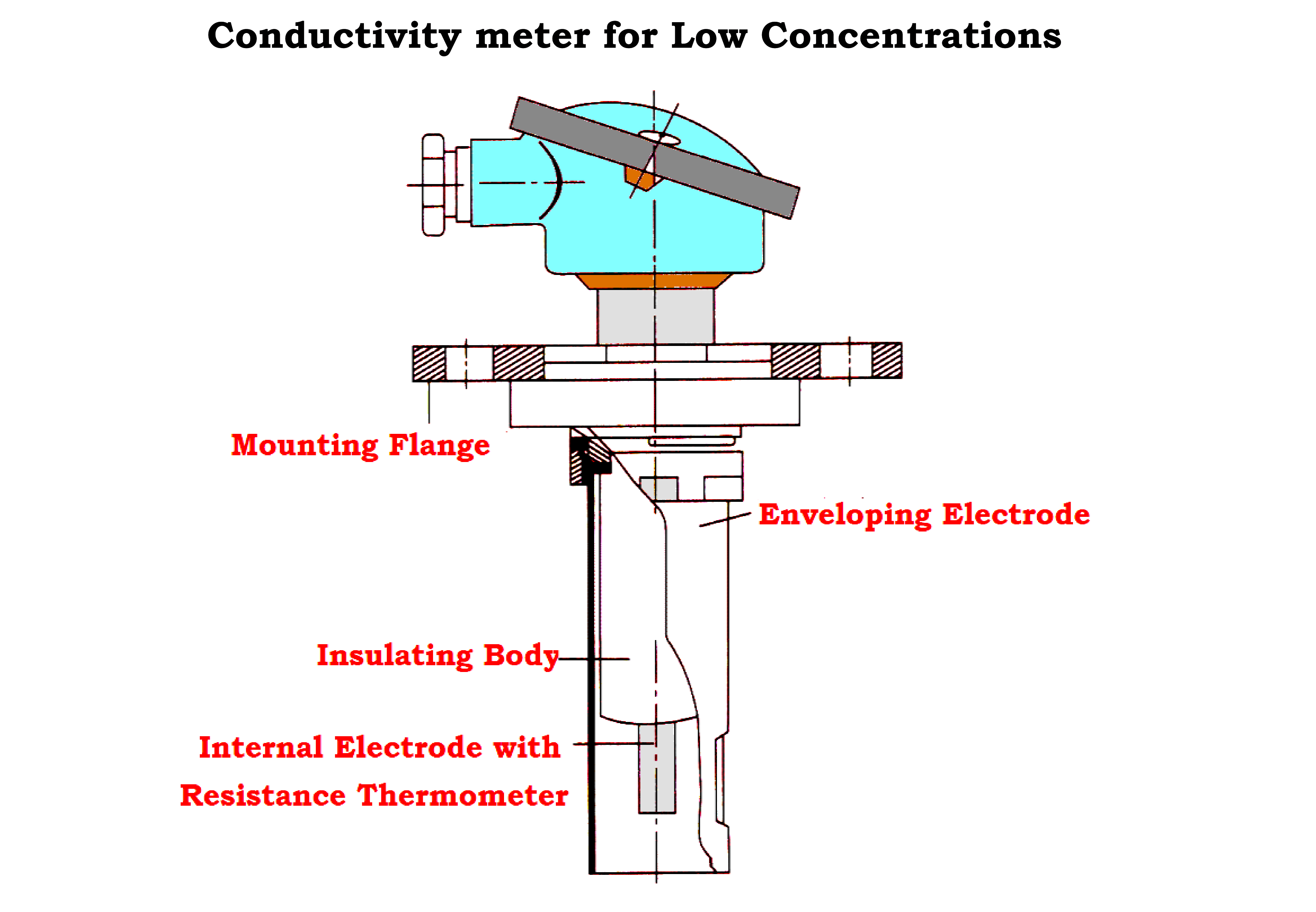
- Detecting Elements for Low Concentrations.
- The electrodes are arranged concentrically.
- The external enveloping electrode is connected to the assembly fitting and earthed via the piping or tank.
- The measurement is not affected by wall effects.
- The internal electrode is insulated and surrounds a resistance thermometer for temperature correction.
- The assembly for very low concentrations has a somewhat thicker internal electrode compared to that for low concentration assembly.
- To avoid polarization effects, one applies an ac voltage in the measurement circuit as shown in the Figure below
- This assembly is usually mounted in a flow chamber but immersion mounting is feasible.
- For pressures exceeding 10 kg/cm² a pressure, a reducer must be provided.
For medium concentration assembly, a plastic sleeve consists of four annular platinum-plated electrodes as shown in the Figure below
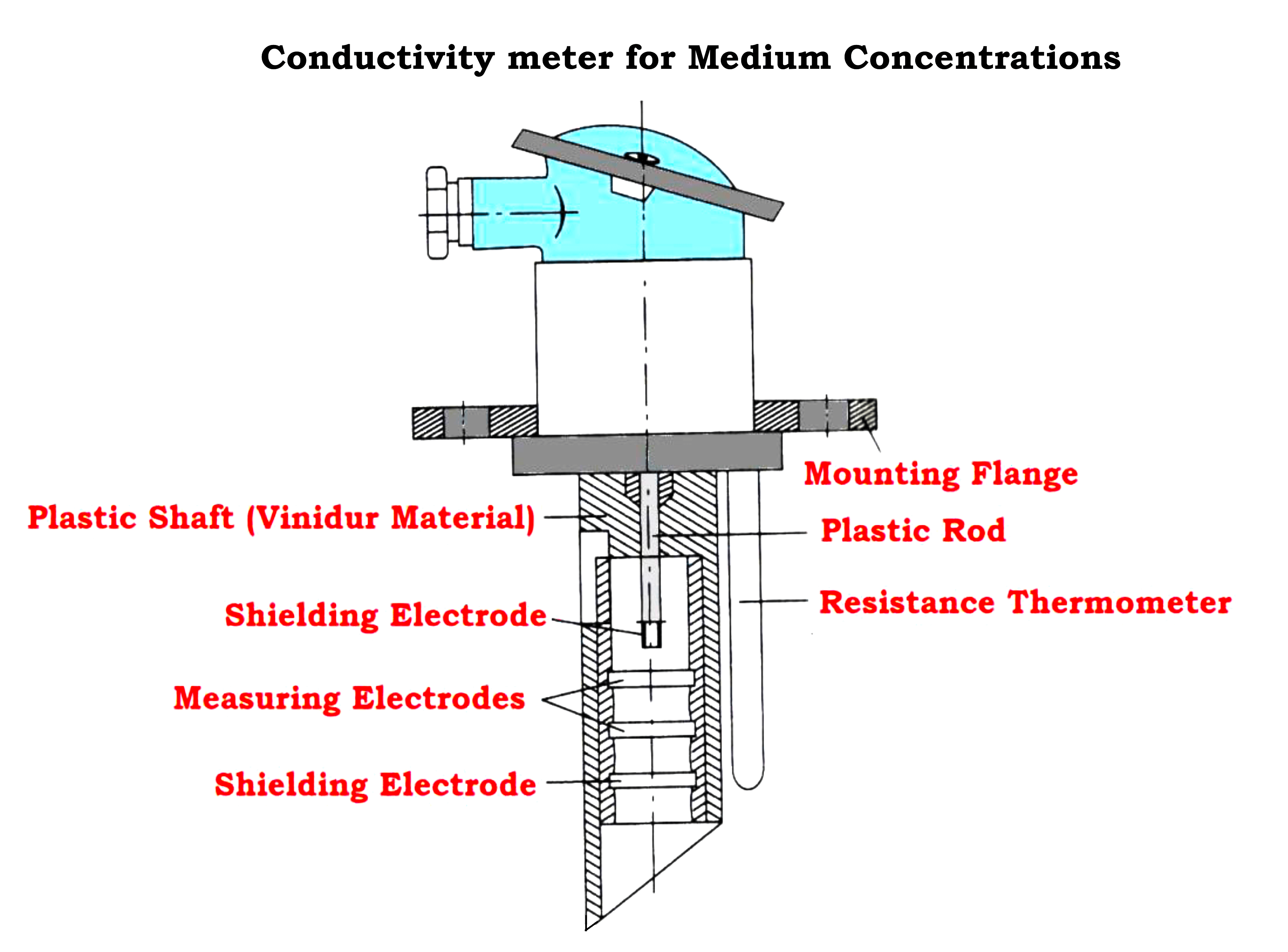
- The two shielding electrodes are electrically connected they regulate the electric field.
- The resistance thermometer allows temperature correction within a protecting tube alongside the electrode sleeve.
- The corresponding electric circuit is shown in the Figure below.
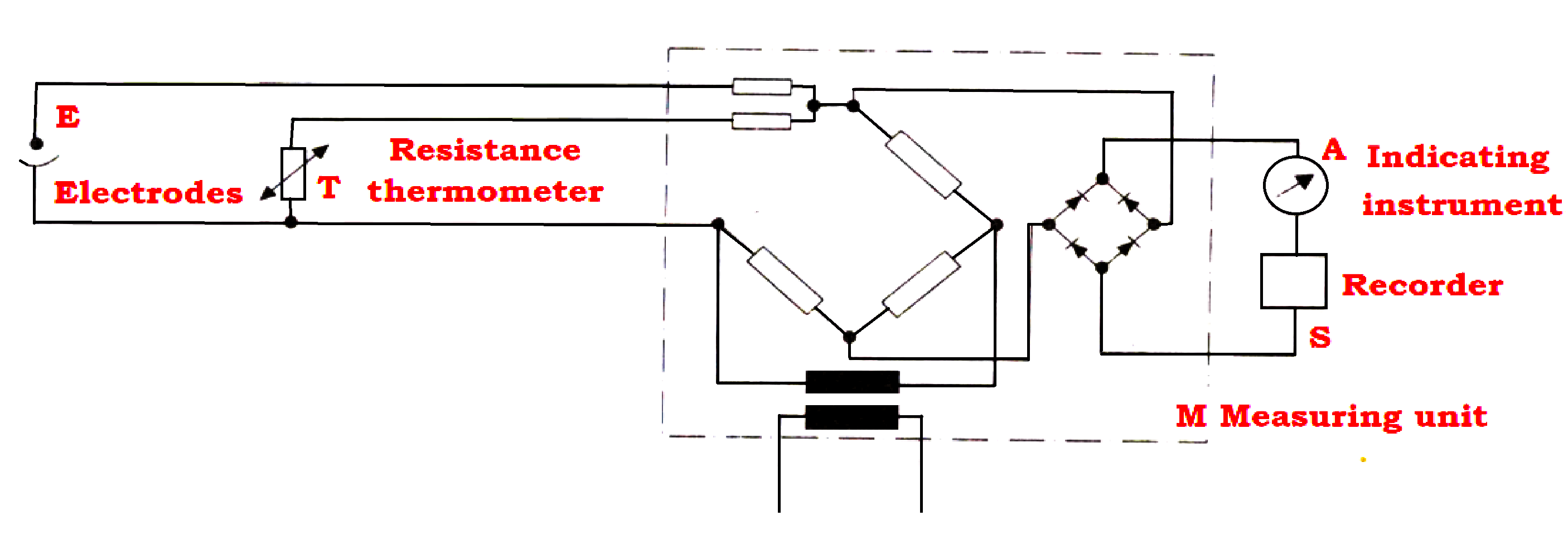
This type of assembly, suitable for pressures up to 1 kg/cm² is usually mounted in a flow chamber.
Measures to be considered:
- The conductivity is influenced by the number of salts and by the content of dissolved gasses such as NH and CO₂ and hence is to be eliminated from the sample.
- Generally, the elimination of NH3 is done by passing the sample through a filter filled with strong acidic cation-exchange resin.
- The CO₂ is then eliminated by passing nitrogen through the sample.
Hydrogen ion Concentration (pH)
- Since, pure water is tasteless, odorless, and neutral.
- But natural and commercial waters are not pure.
- Because of ionization, water is considered the most universal solvent.
- Either OH- or H+ ions will predominate, causing either an alkaline or acidic condition.
- pH scale devised to measure the intensity of acidity or alkalinity of a solution. An exact definition of the pH number is that it is the logarithm of the reciprocal of the hydrogen ion concentration (grams per liter).
pH = log10 (1/H) = – log10 (H+)
- The pH value is a measure of the strength of an acid and base, to the neutral character of a solution.
- The law of mass action applies to all chemical reaction states for the dissociation of water, H+ x OH– = 10-14 K (at 25°C).
- Just as the temperature is measured in degrees, so is acidity or alkalinity measured in pH values from 0 to 14.
- pH 1 is strongly acidic, pH 14 is strongly basic, and pH 7 is a neutral solution.
- pH 5 is 10 times as acidic as PH 6.
- pH 4 is 10 times as acidic as pH 5 and 100 times as acidic as pH 6.
For instance.
- If H+ is 10-9 then OH– is 10-5 grams ions per liter, (H+ x OH– = 10-14=K) and pH 9 designates the solution which, of course, is alkaline as OH ions predominate.
Analysis of Dissolved Oxygen
- The dissolved oxygen present in feed water is a corrosion accelerator.
- The unit used to mention dissolved oxygen is ppm.
- Analysis to quantify dissolved oxygen can be done either by laboratory method or by online analysis.
- The chemical titration method is normally used to find out the quantity of dissolved oxygen from the sample collected and immediately presented for the test.
- The validity of measurement will be under suspicion as the delay causes the absorption of oxygen from the atmosphere into the sample.
- Special portable kits are used to carry out the testing on the spot. It is always better to use analyzers that can test online.
- Two such analyzers are discussed briefly here.
Wallace and Tiernan Dissolved Oxygen Analyzer
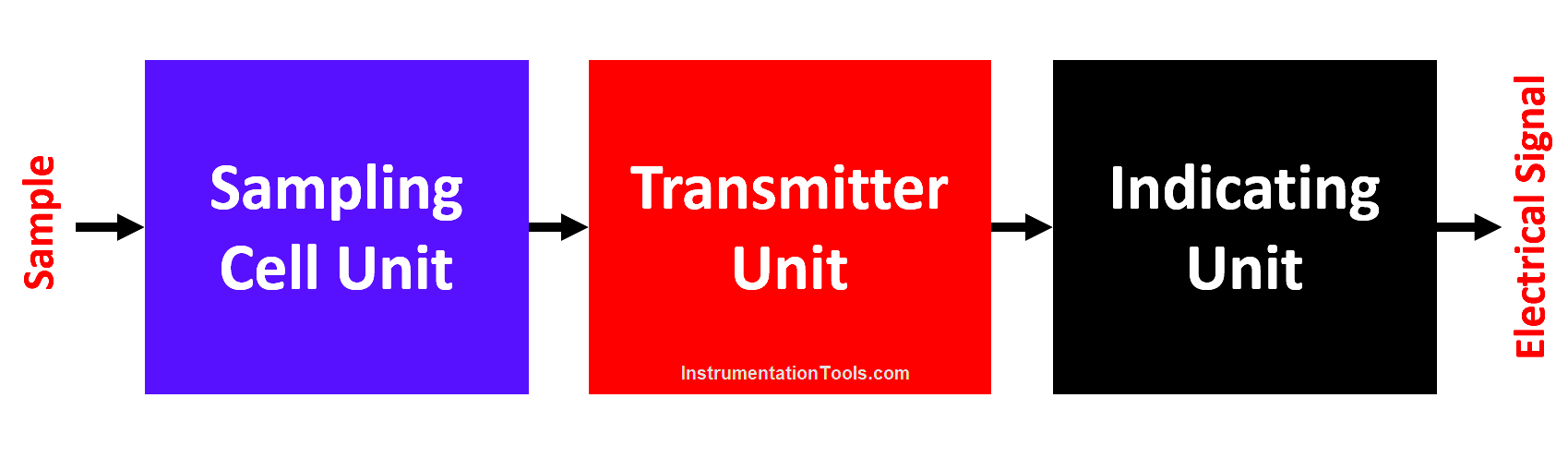
A simplified block diagram of the Wallace and Tiernan dissolved oxygen analyzer is shown below
- The concentration of dissolved oxygen in boiler water is measured by electrochemical analysis in the analyzer.
- The electrochemical analysis is continuous and automatic.
- The analyzer can be divided into two units called the sampling cell unit and the transmitter indicator unit.
- The sampling unit consists of a reference electrode, platinum measuring electrode, and calibration cell for measuring and controlling sample flow, pressure, temperature, and conductivity.
- The transmitter-indicator unit consists of electronic circuits required for measurement, transmission, and calibration.
- The indicating meter or recorder is normally calibrated in terms of ppb or parts per billion.
- Ranges may be from 0 to 25 and 0 to 50 ppb with multiplication factors of 1 and 10. That means, in addition to 0 to 25 and 0 to 50 ppb ranges another set of ranges 0 to 250 and 0 to 500 ppb are made available.
- The nominal output voltage in the range of 0-5 mV can be converted into standard signals of either 4-20 mA or 1 to 5 V DC.
Kathrometer
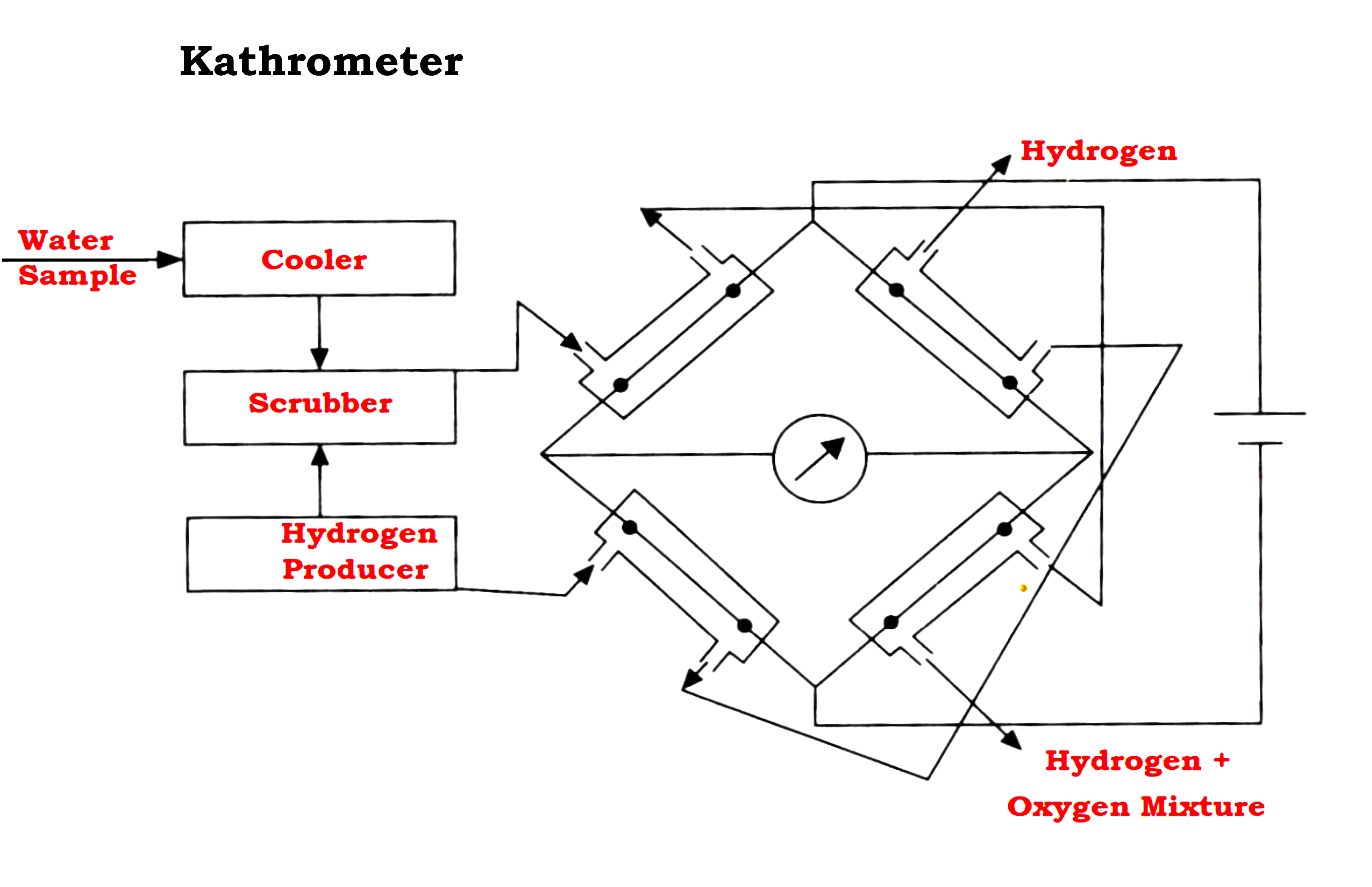
- The Kathrometer consists of four major parts: Cooler, Scrubber, Hydrogen Producer, and Wheatstone bridge circuit.
- When the water sample is cooled and scrubbed by hydrogen, the dissolved oxygen present in the water gets displaced to form a mixture of hydrogen and oxygen.
- The Wheatstone bridge circuit is used to measure the amount of dissolved oxygen. The heated wires of the bridge circuit are exposed to hydrogen in two opposite arms and to a hydrogen-oxygen mixture in the other two arms.
- The unequal cooling of the heated filaments makes the bridge imbalanced.
- The unbalanced voltage can be calibrated to indicate the dissolved oxygen.
If you liked this article, then please subscribe to our YouTube Channel for Electrical, Electronics, Instrumentation, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Turbine and Compressor System
- Steam Circuits in Power Plants
- Furnace Draft Control System
- Turbine Steam Pressure Control
- Working Principle of Steam Ejector