Calibration and Preventative Maintenance
Calibration: A comparison of two instruments or measuring devices one of which is a standard of known accuracy (traceable to national standards) to detect, correlate, report or eliminate by adjustment, any discrepancy in accuracy of the instrument measuring device being compared to the standard.
Calibration Programs Required by Regulatory Authorities
- “Automatic, mechanical, or electronic equipment or other types of equipment, including computers, or related systems that will perform a function satisfactorily, may be used in the industries like power plant, oil & gas, cement, manufacture, processing, packing etc. If such equipment is so used, it shall be routinely calibrated, inspected, or checked according to a written program designed to assure proper performance.
- Maintenance at appropriate intervals to prevent malfunction & shall be “preventative” not “reactive” maintenance.
Calibration requirements for Laboratory Instruments
- Specific Directions
- Schedules
- Limits of accuracy & precision
- Remedial Actions
- Systems to prevent usage of instruments failing calibration
• “Control, weighing, measuring, monitoring and test equipment that is critical for assuring the quality of intermediates or APIs should be calibrated according to written procedures and an established schedule.
Each Manufacturing / Process Area:
- Written calibration procedures that use traceable calibration standards or calibration equipment.
- Preventive maintenance procedures and / or referenced manuals
- Qualified individuals (having the appropriate education, training, background and experience) responsible for calibrating & maintaining instrumentation
- Second person check of all calibration and maintenance
- Qualified individuals responsible for monitoring the calibration and maintenance program.
- Ensure the calibration program and procedures are reviewed and approved by Quality
Instrument / Equipment Master List
- System for identification of all Master equipment related to instrumentation in a manufacturing/process area or laboratory
- Include instrumentation details (serial number, model number & location)
- If automation components are tracked separately through the configuration management then it is not necessary to include (must verify)
- Procedures must exist that identify the calibration and maintenance requirements for each instrumentation / equipment on the master list
Retired Equipment
- Records pertaining to retired / obsolete equipment must be kept according to company’s records retention procedures
- Records should include date the unit was retired, person responsible and the reason for retirement / discard
Instrument Identification & Calibration Status
- Each instrument given a unique identifier
- Instrumentation details associated with this number must be documented and available (e.g. serial number, model number, location, etc.)
- Each instrument should be labeled with the unique identifier
- Calibration status of each instrument , the date of calibration, the next calibration date and the identification of person performing calibration should be readily available
- Appropriate systems to document calibration status include calibration logs, MAXIMO, and calibration stickers
- System must be in place to prevent use of an instrument that is not qualified, unusable due to damage or malfunction, or has exceeded its established calibration interval
- System must be in place that identifies instruments that do not require calibration to be performed beyond the original or factory calibration to distinguish from those instruments that do require scheduled calibrations
⇒Documentation required for excluding equipment
Traceability of Standards / Calibration Equipment
- Calibration reference standards / calibration equipment shall be traceable to national standards and be accompanied by certificates of traceability / analysis
- If recognized standards are not available, an independent reproducible standard may be used
- The calibration tolerance of a given standard should be as tight or tighter than the tolerance of the instrument to be calibrated
- A procedure must be in place to ensure tracking and monitoring of standard’s expiration date and re-calibration / re-certification requirements
- Re-calibration records must be retained
Instrument Calibration Tolerances
Instrument calibration tolerance limits should be established so problems are identified and corrected in a timely manner
When assigning tolerances, considerations given to:
- Capability of the instrument being calibrated (what the manufacturer/OEM claims the instrument can achieve).
- Parameters at which the instrument operates (ex: if testing accuracy of + 0.5% is required, the instrument calibration tolerances should be <0.5%)
- Work environment – environmental conditions can affect the performance of the instrumentation
Practice of using “Alert” & “Action” levels
“Alert” Tolerance (“Adjustment Limit”)
- Related to instrument performance
- Level at which the instrument is adjusted back into range
- Not required – an industry “best practice”
“Action” Tolerance (“Calibration Limit” or “Out-of-Tolerance”)
- Tied to process performance
- Level at which the potential for product impact exists
- Possible atypical investigation or reporting is required
Deviations beyond “Alert” level but not at “Action” level would not require investigation – May require adjustment, changes to PMs
Setting of “Alert” and “Action” levels should be described in SOPs, be defendable and have Quality review and approval
Calibration and Maintenance Frequencies
May be determined for individual instruments or groups of instruments (similarity of construction, reliability, and stability)
Considerations when determining calibration frequency:
- Accuracy of the measurement / instrument range
- Consequences of incorrect value caused by out of calibration
- Extent & criticality of use of the instrument & tendency to wear and drift
- The manufacturer’s/OEM’s recommendations
- Environmental & physical conditions (temperature, humidity, vibration)
- Previous calibration records, history of calibration problems & repair history
- Frequency of calibration checks prior to use or in-between intervals
- Redundant / back-up systems (provides secondary source of information available from other calibrated primary instruments)
- Results of Qualification studies
- Process requirements
- Availability of built-in / automatic calibration checks
Changes to frequency must be approved per change control SOPs
References to specific instrument procedures listed in compendium (USP, BP or EP) for calibration tests for specific laboratory instruments
Calibration time “windows” should be established around calibration due dates
Policy for Calibration and Maintenance Intervals & Schedules Include:
- Extending Intervals
- Reducing Intervals
- Maximum Intervals
Maintenance Requirements
- Manufacturer’s/OEM’s recommendations
- Parts that wear: gaskets, seals & bearings
- Parts requiring periodic replacement: filters, belts & fluids
- Parts requiring periodic inspection and cleaning
- Parts requiring periodic adjustments, tightening and lubrication
Calibration and Maintenance Procedures
- Shall include specific directions and limits for accuracy & precision
- Shall include guidance for remedial action when accuracy & precision limits are not met
- Normally provided in the manufacturer’s/OEM’s manuals
- Some compendial requirements exist for some specific laboratory instrumentation
- Performance checks (e.g. system suitability, daily balance performance checks) are NOT suitable substitutes for regularly scheduled calibrations
Each calibration & maintenance procedure should include the following:
- Identification of department responsible to perform the calibration or maintenance
- Step-by-step calibration instructions, reference to appropriate calibration procedures or instrument manuals
- Methods for preventive maintenance or reference to appropriate instrumentation manuals
- Calibration equipment used in the calibration (e.g. spectroscopy filters, voltmeters, digital thermometers, etc)
- Calibration parameter and tolerance ( ± )
Each calibration & maintenance procedure should include the following:
- Required environmental controls or conditions, where appropriate
- Provisions for adjustments, if needed
- Requirement for recording actual measurements before (“as found”) and after adjustment or preventive maintenance (“as left”)
- Actions to be taken if instrumentation cannot be calibrated (e.g. contact appropriate service people, label and remove from service)
- A step to record all calibration & maintenance activities.
Note : “as found” recordings are not required where routine performance checks are available to provide evidence indicating instrument is operating properly and is suitable for use.
Use of Contractors / Vendor Service Personnel
- Must have a procedure for approving contractor activities
- Contractors must have appropriate training / qualification
- Responsible to review & approve the contractors calibration and maintenance procedures.
- Responsible to perform actions or steps not part of the contractors procedures.
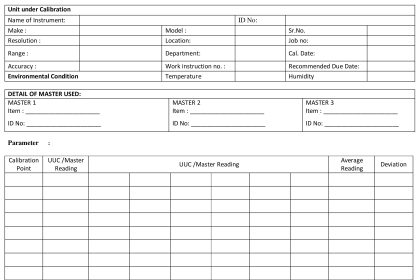
Calibration & Maintenance Records
- All calibration records must be retained per document retention procedures
- Should include “as found” measurements, results of adjustments (“as left”) and appropriate review & approval of all results
- Tolerance or limit for each calibration point
- Identification of standard or test instrument used
- Identification of persons performing the work and checking the results with dates
- Review must ensure the approved activities have been completed and all results have passed the established acceptance criteria
- Periodic review of historic calibration & maintenance data to evaluate appropriateness of established frequencies
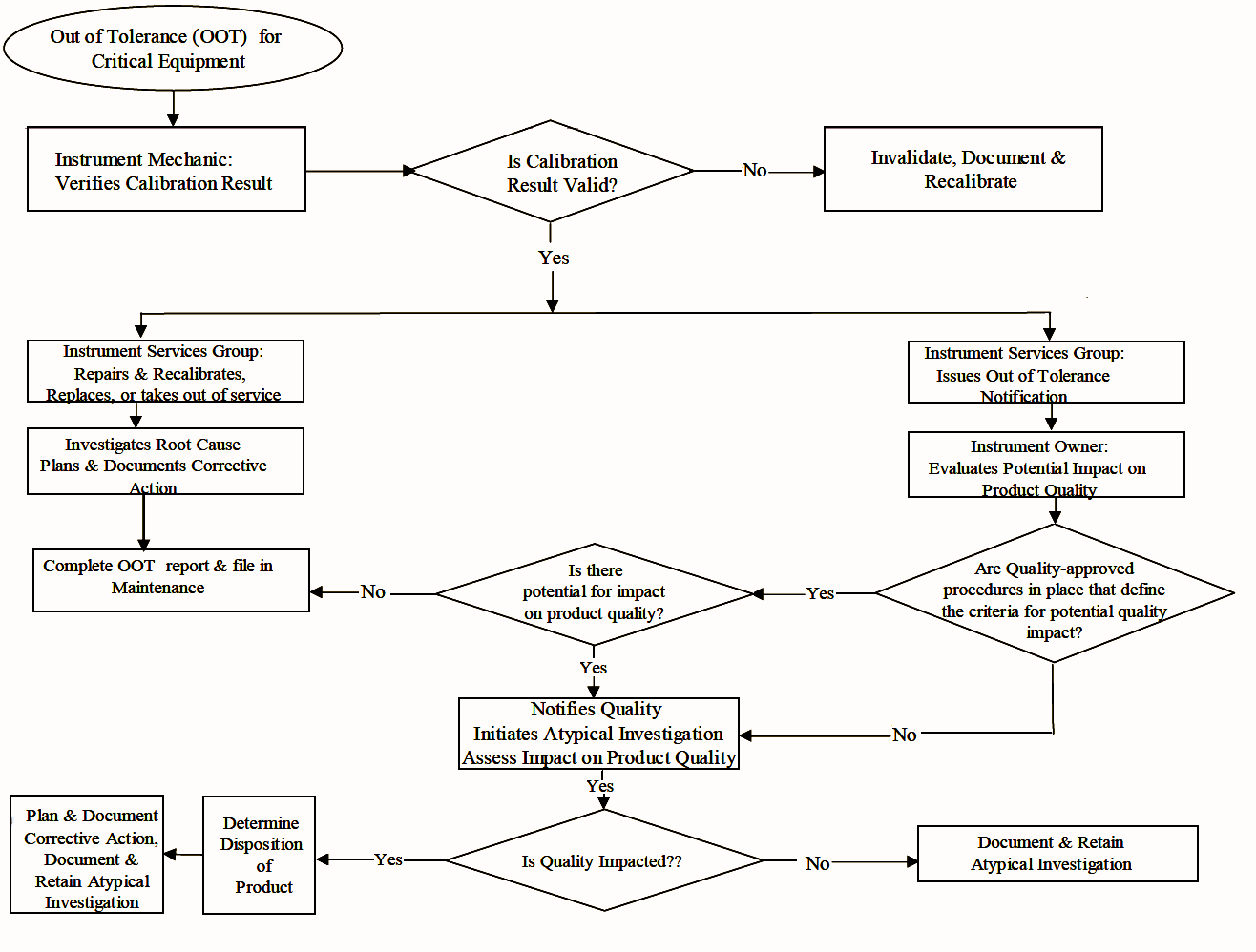
Procedures for Out-Of-Tolerance Calibration Results
Actions required when critical instrument found outside of established instrument calibration limits (“Action Limit”,Out-Of-Tolerance, OOT):
Critical Instrumentation: Any instrument employed in the production of bulk pharmaceutical chemicals, drug or biologics product that controls and/or is used to measure a parameter that affects the validation state of the product (Critical Process Parameters) and any other quality parameter designated as such. Also includes laboratory instrumentation used to measure conformance to component and/or product specifications.
Instrument Mechanic/Technician:
- Immediate review of calibration data to verify validity of OOT
- Notification to supervisor or responsible person
Instrument Services :
Corrective Action to the instrument ASAP (repair, re-calibration, replacement or removal from service)
Investigation and documentation into root cause of OOT
- Historical review of instrument performance & factors relevant to setting calibration frequency
- Corrective Actions to prevent recurrence
- Re-calibration after maintenance or repair
OOT Notification issued to instrument owner (after results validated)
OOT Notification should include current calibration data, magnitude of OOT error, and date of last successful calibration
Instrument Owner :
Evaluate impact of OOT on product quality.
Impact evaluation based on SOP which is approved by Quality and defines how / when Quality will be notified.
Ex. of OOT with impact on quality:
- Instruments used in acceptance / rejection of materials and product
- Control / monitoring of critical process parameters
- Process controls affecting the final product quality or yield
- Instruments required by regulation (e.g. gauges, RH monitors, etc.)
- Calibration reference standards
Needs complete understanding of the process requirements for the parameter measured by the OOT instrument
Evaluate magnitude of error in the “As Found” OOT vs. process requirements to determine quality impact
Ex. of OOT with no impact on quality:
⇒A temperature transmitter is reading 0.2 Deg C low and is OOT for that instrument. There is no potential for quality impact if:
– Process requires temperature reading accuracy of +/- 1 DegC –
– Measures a range / CPP with max. 85 Deg C and process normally runs at 75 Deg C.
- Decisions regarding impact on quality must be documented, approved by Quality and documentation retained
- OOT events should be tracked and trended to identify problem instruments
- Historical information on OOT events should be readily retrievable
Change Control Management
- Changes to calibration tolerances, frequency, procedures, addition to / deletion from calibration program, changes in location and different replacement parts should be documented in a change control program
- May also require re-calibration, re-execution / revision of instrument’s Installation Qualification (IQ), Operational Qualification (OQ) or Performance Qualification (PQ)
- Update of qualification records: drawings, parts list, etc.
- Appropriate review and approval by responsible departments and Quality
FLOWCHART :
Credits : Sally Wolfgang Manager, Quality Operations Merck & Co., Inc.






Can you please assist with sending me a template for instrument preventive maintenance?
hello, Can you please assist with sending me a template for instrument preventive maintenance (routine and major) ?
Thank you.
Hi, Can you suggest Which procedure need to follow first during routine usage of instrument as per guidlene ( preventive maintenance or calibration)