Boiler Feed Water Treatment
- The impurities present in boiler feed water can have many bad effects on water tube boilers and steam turbine blades.
- In this article, we will learn the treatment of boiler feed water.
- The condition of feed water is studied and known by making measurements for conductivity, pH, and dissolved oxygen.
- The total treatment boiler feed water required is broadly classified into two categories.
- External Treatment is to be carried out before supplying water to the boiler.
- Internal Treatment is to be carried out after the water is let into the boiler.
External Treatment of Boiler Feed Water
- External treatments such as Mechanical, Thermal, and Chemical treatments are possible.
- Mechanical treatment includes Sedimentation, Filtration & Interior painting.
- Thermal treatment includes De-aeration & Evaporation.
- Chemical treatment includes Precipitation using prepared reagents and exposure to a zeolite to obtain ion exchange.
- The ion exchange process is done prior to the water entering the boiler.
- Precipitation is possible in both internal and external treatment.
Filtration
- Coagulation is carried out with the addition of special chemicals such as Aluminum Sulphate with suspended solids and turbidity.
- The coagulated matter settles down in the clarifier which can be removed easily.
- Positive filtration is essential for higher turbidity of the clarified effluent.
- Gravity filters and pressure-type filters are used for positive filtration, generally, Pressure type filters are preferred.
- A granular material such as sand is used in the filtration process.
- The pressure difference across the granular medium represents the accumulation of solids.
- The bed has to be back washed once the limit reaches. Organic compounds can be removed with the help of activated carbon and residual chlorine through the chlorination process.
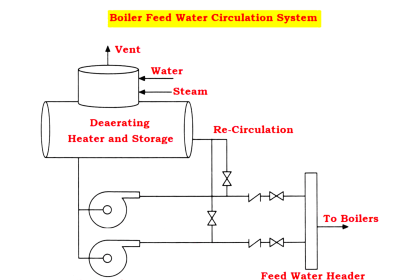
De-aeration
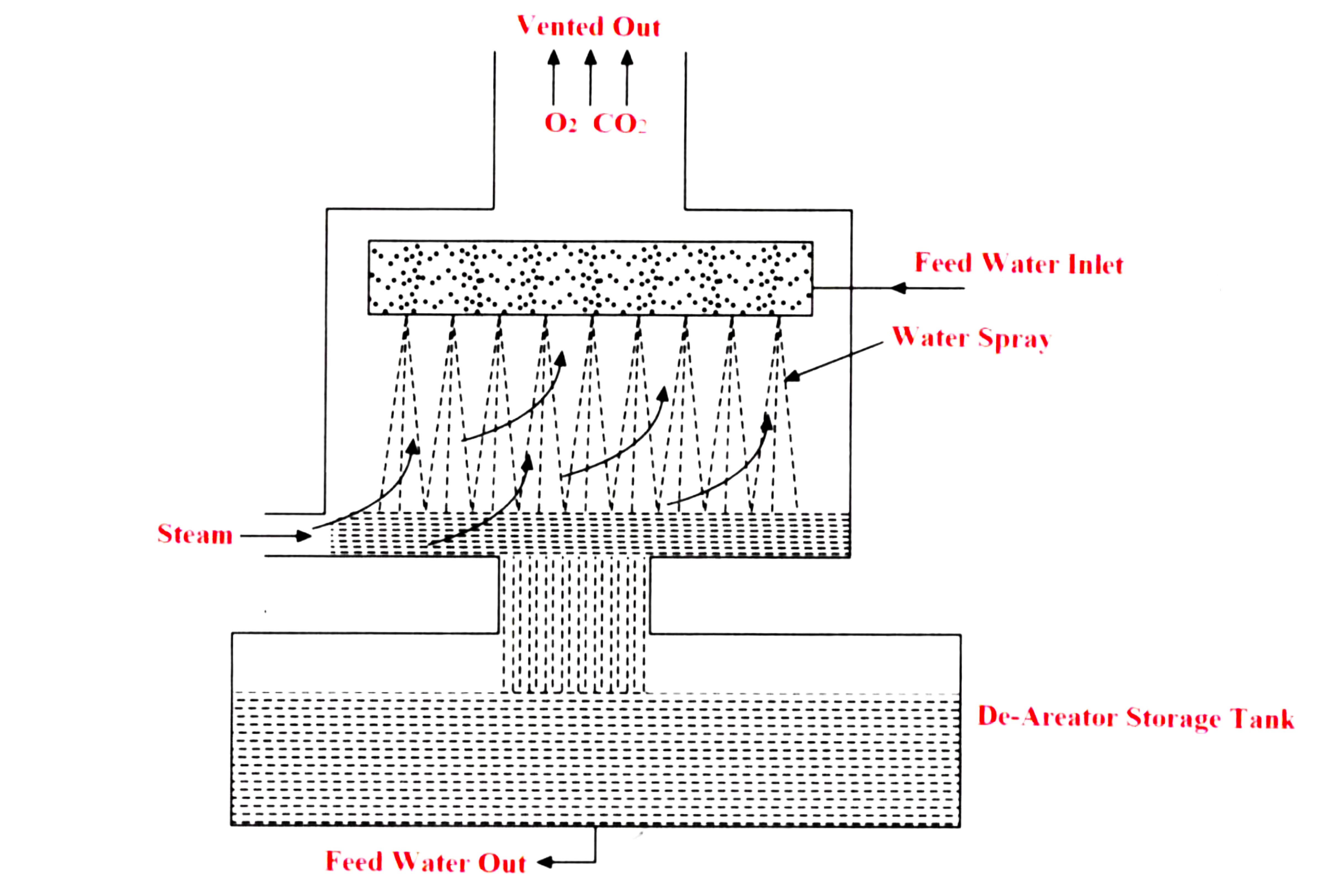
- The de-aerator with a storage tank is shown in the above figure
- Removal of dissolved gases like oxygen and carbon dioxide by heating and agitating is done by De-Aeration or degasification process.
- With the increase in temperature, the solubility of these gases becomes less and almost zero at the boiling temperature.
- The heating can be done by the extracted steam from the turbine. After heating up, the water is sprayed from the top so that the gases can escape through the vent and the water gets collected in a storage tank.
Precipitation
- The precipitation process is used to remove dissolved salts from feed water.
- Lime, Calcium hydroxide and soda ash are used to remove calcium and magnesium salts in the hot lime-soda process of precipitation.
- The resultant products, namely, calcium carbonate and magnesium hydroxide which are insoluble in the water get precipitated and settle down in the bottom of the vessel.
Ion Exchange Process
- In a water solution, some materials will exchange their base radicals with anions.
- For softening water, the exchange of calcium for sodium is accomplished. Sodium zeolite [Na₂ (Al₂Si₂0g) or Na₂Z] is used for this purpose.
- Organic zeolite containing no silica is also used for ion exchange purposes.
- Sodium zeolite (Na₂Z) and organic zeolite (H₂Z) have the ability to exchange one ion for another, hold it temporarily, and give it up to a string regenerative solution.
De-Mineralization
A diagrammatic representation of the Demineralization plant is shown in Figure
Contents
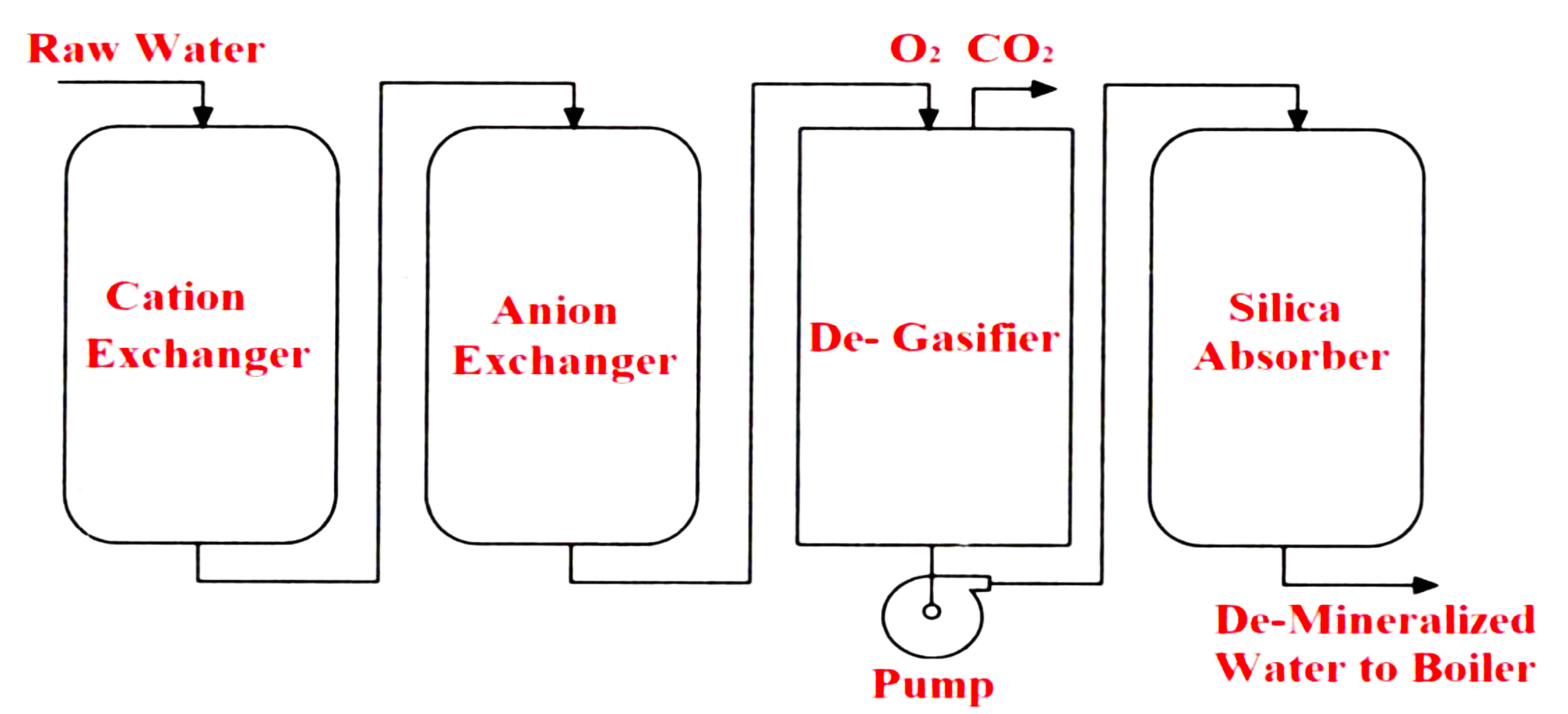
- De-Mineralization is the process by which the dissolved salts present in feed water are removed by ion exchange.
- Anion resin (Na₂Z) and cation resin (H₂Z) is used for ion exchange purpose.
- The hydrogen ion is exchanged for the cation calcium, sodium, and magnesium when H₂Z is used.
- Anion resin absorbs the anion chloride, nitrates, and sulfates.
- The ion exchange process is reversible and hence the resins can be restored by regeneration.
- Due to condenser leakage and the presence of metallic ions in iron and copper pipelines, the treated water may pick up impurities.
- the picked up impurities in condensate water present are eliminated by the condensate polishing process
- Mixed bed units with both Cation and Anion resins called De-Mineralizing vessels are used for this purpose.
- These units remove impurities such as suspended solids and dissolved salts.
Internal Treatment of Boiler Feed Water
- Treating the water within the boiler is known as internal treatment.
- Even after external treatment of boiler feed water some dissolved solids are escaped and accumulated.
- This internal treatment is a slow process, hence the concentration of dissolved solids may rise in the boiler feed water.
- Unless the concentrated water is De-concentrated or blown down regularly, foaming and carryover take place encouraging scaling, corrosion, and Embrittlement.
- The blow-down operation is intermittent or continuous.
- The amount of blowdown depends on allowable solids concentration.
- It is always expressed as below:
% Blow down = (Quantity of water blown down/Quantity of feed water admitted) x 100%
- Chemical reagents are periodically added to the boiler water.
- An electrolytic apparatus having a ‘sacrificial anode’ is installed in the boiler drum.
- The special metallic anode is consumed in boiler protection against scaling and oxygen corrosion.
- Some of the chemicals employed and the purpose for which they are employed are given in Table 1
Table 1 Chemicals used for internal treatment.
| Chemical | Purpose |
| Sodium Hydroxide | To establish a pH of more than 7, and to precipitate magnesium content |
| Sodium Carbonate | To precipitate calcium |
| Sodium Aluminate | To coagulate finely divided precipitates and to precipitate calcium and magnesium contents. |
| Sodium Phosphates (Mono,Di,or Tri sodium) | To precipitate calcium in a form favorable to riddance through blow down |
| Sodium Sulphate | To complete de-oxidation |
| Colloids | Protective colloids such as tannin; and reactive organic forms make a fluid sludge of suspended particles. |
| Acid | To control alkalinity |
Steam Purity
- The steam quality delivered by the boiler is based on the purity and chemical parameter of boiler feed water such as pH, TDS, and so on.
- The quality steam means that it is at the required temperature and pressure in addition to having the least carryover and least boiler salt concentration.
- Impure steam causes scaling, corrosion and Embrittlement.
- Scrubbers and steam washers are used in the drum to get the required steam purity.
- Steam purity can be determined by measuring the conductivity of the condensed sample using conductivity meters.
- Electrical conductivity is considered the representative measure of impurity in the water.
- The summary of feed water treatment is shown in table 2.
Table 2 Summary of feed water treatment
| Impurity | Effect | Kind of treatment |
| 0₂ | Corrosion | Chemical De-oxidation, Thermal De-aeration |
| Co₂ | Corrosion | Thermal De-aeration |
| Ca, Mg, Salts | Scale | External Softening Internal Softening |
| Fe | Scale | Ion Exchange |
| Sio₂ | Carry Over Scale | De-ionization |
| Na Alkalinity | Embrittlement, Foam | Acid Neutralizer |
| TDS | Priming, Foam | De-concentration |
| Turbidity | Sludge Sediment | Coagulation, Filtration, Sedimentation, |
| Oil | Foam | Coagulation, Filtration, Surface Blow-Off |
If you liked this article, then please subscribe to our YouTube Channel for Electrical, Electronics, Instrumentation, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Turbine Bypass System
- Boiler Light-Up Sequence
- Deaerator Control System
- Furnace Draft Control System
- Water and Steam in Power Plant