Specific gravity is defined as the ratio comparing the weight of any liquid to the weight of an equal volume of water. The specific gravity of pure water is 1.000. Lead-acid batteries use an electrolyte which contains sulfuric acid. Pure sulfuric acid has a specific gravity of 1.835, since it weighs 1.835 times as much as pure water per unit volume.
Since the electrolyte of a lead-acid battery consists of a mixture of water and sulfuric acid, the specific gravity of the electrolyte will fall between 1.000 and 1.835. Normally, the electrolyte for a battery is mixed such that the specific gravity is less than 1.350.
Specific gravity is measured with a hydrometer.
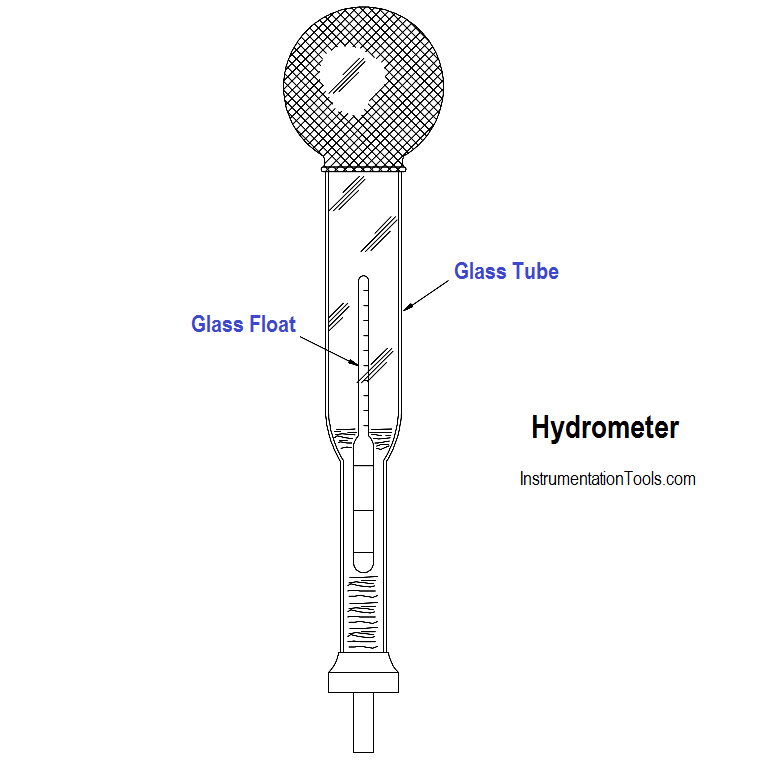
Figure 1 : Simple Hydrometer
A simple hydrometer consists of a glass float inside a glass tube, as shown in Figure 1.
The hydrometer float is weighted at one end and sealed at both ends. A scale calibrated in specific gravity is positioned lengthwise along the body of the float. The float is placed inside the glass tube, and the fluid to be tested is drawn into the tube.
As the fluid is drawn into the tube, the hydrometer float will sink to a certain level in the fluid. The extent to which the hydrometer float protrudes above the level of the fluid depends on the specific gravity of the fluid. The reading on the float scale at the surface of the fluid is the specific gravity of the fluid.