Level measurement systems that use differential pressure ΔP as the sensing method, are by their very nature affected by temperature and pressure.
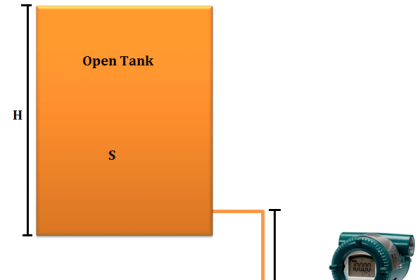
Recall that the measured height H of a column of liquid is directly proportional to the pressure P exerted at the base of the column and inversely proportional to the density ρ of the liquid.
H α P/ρ
Density (mass per unit volume) of a liquid or gas is inversely proportional to its temperature.
ρ α 1/T
Thus, for any given amount of liquid in a container, the pressure P exerted at the base will remain constant, but the height will vary directly with the
temperature.
H α T
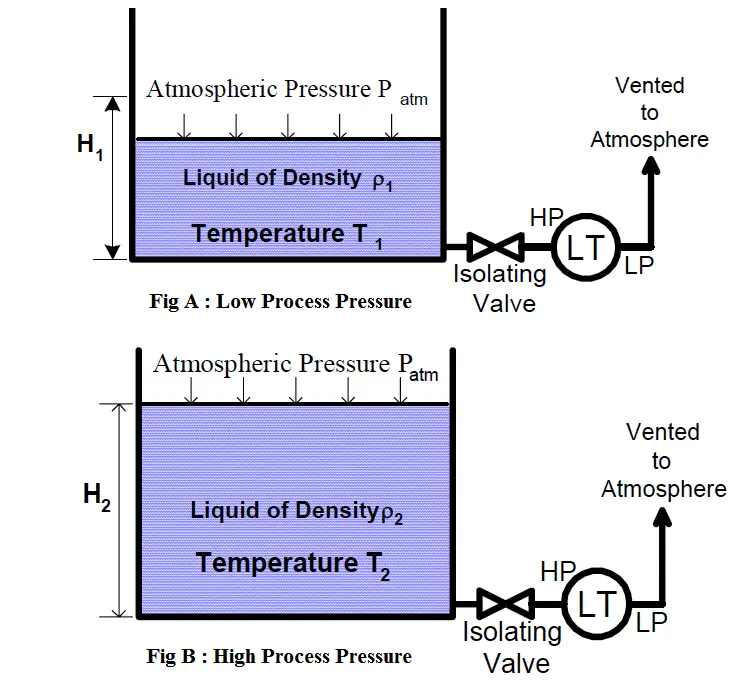
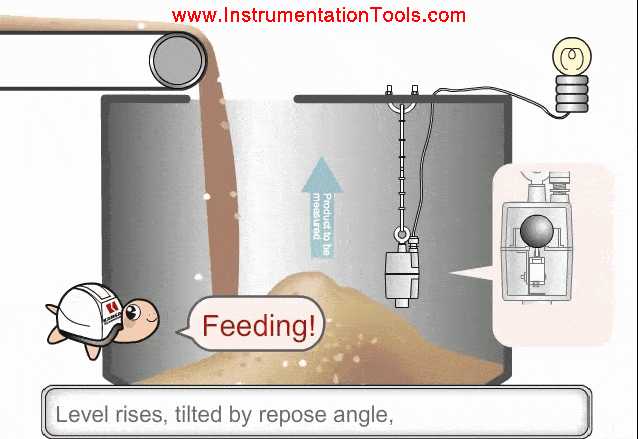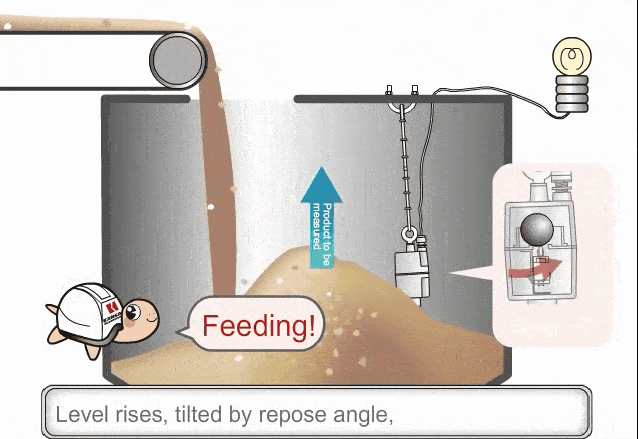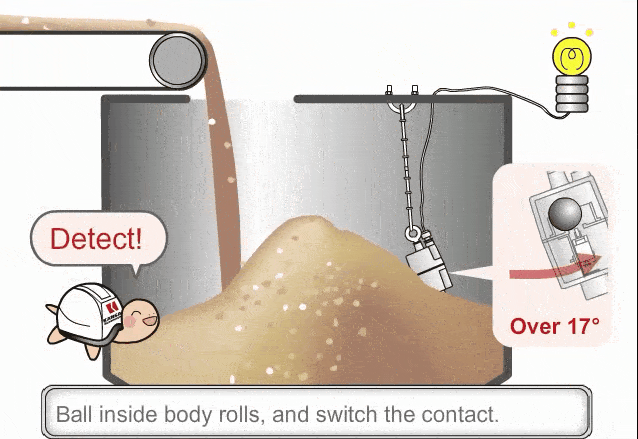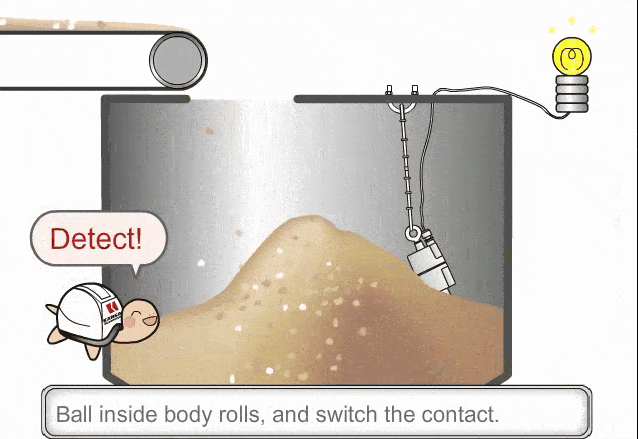
Consider the following scenario. A given amount of liquid in a container [figure (a)] is exposed to higher process temperatures [figure (b)].
As the amount (mass) of liquid does not change from figure (a) to (b), the pressure exerted on the base of the container has not changed and the indicated height of the liquid does not change. However, the volume occupied by the liquid has increased and thus the actual height has increased.
The above scenario of figure is a common occurrence in plant operations. Consider a level transmitter calibrated to read correctly at 75 DegC.
If the process temperature is increased to 90 DegC, the actual level will be higher than indicated.
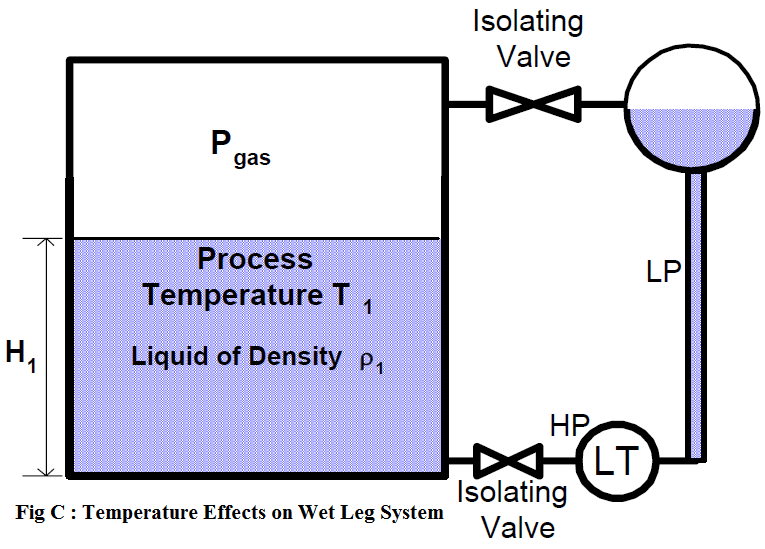
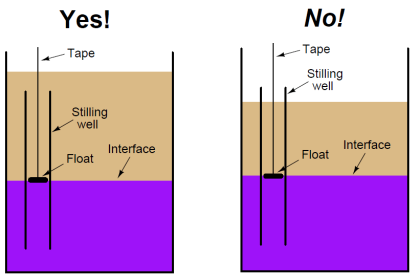
The temperature error can also occur in wet-leg systems (figure c).
If the reference leg and variable leg are at the same temperature that the level transmitter (LT) is calibrated for, the system will accurately measure liquid level. However, as the process temperature increases, the actual process fluid level increases (as previously discussed), while the indicated measurement remains unchanged.
Further errors can occur if the reference leg and the variable (sensing) leg are at different temperatures. The level indication will have increasing positive (high) error as the temperature of the wet reference leg increases above the variable (process) leg.
As an example, consider temperature changes around a liquid storage tank with a wet leg. As temperature falls and the wet leg cools off, the density of the liquid inside it increases, while the temperature in the tank remains practically unchanged (because of a much bigger volume and connection to the process).
As a result the pressure of the reference leg rises and the indicated level decreases. If it happens to the boiler level measurement for a shutdown system it can even lead to an unnecessary reactor trip on boiler low level. However, high-level trips may be prevented under these circumstances. In an extreme case the wet leg may freeze invalidating the measurement scheme completely, but it could be easily prevented with trace heating.
False high level indication can be caused by an increased wet leg temperature, gas or vapour bubbles or a drained wet leg.
A high measured tank level, with the real level being dangerously low, may prevent the actuation of a safety system on a low value of the trip parameter.
The real level may even get sufficiently low to cause either the cavitation of the pumps that take suction from the tank or gas ingress into the pumps and result in gas locking and a reduced or no flow condition. If the pumps are associated with a safety system it can lead to possible safety system impairments and increased probability of resultant fuel damage.
Effect of Pressure on Level Measurement
Level measurement systems that use differential pressure ΔP as the sensing method, are also affected by pressure, although not to the same degree as
temperature.
Again the measured height H of a column of liquid is directly proportional to the pressure P exerted at the base of the column by the liquid and inversely proportional to the density ρ of the liquid:
H α P/ρ
Density (mass per unit volume) of a liquid or gas is directly proportional to the process or system pressure Ps.
ρ α Ps
Thus, for any given amount of liquid in a container, the pressure P (liquid pressure) exerted at the base of the container by the liquid will remain
constant, but the height will vary inversely with the process or system pressure.
H α 1/Ps
Most liquids are fairly incompressible and the process pressure will not affect the level unless there is significant vapour content.




There are other complications from other sources:
1) Change of vessel dimensions with both pressure and temperature (this can be either temporary (plastic deformation which requires a spring term in the equation) or permanent (and varying with time)). This is typically limited to 1 to 2% in metallic vessels at most ambient temperatures but may be as high as 10% in polymer vessels or high temperature services. This really becomes noticeable in systems such as boiler steam drums and distillation reboilers where only a small percentage of the total volume is measured and may result in levels varying from 100 to 0% as the system is pressurized, even without heat applied.
2) Effects of change of state of the contents of the vessel, particularly an issue for steam and cryogenic systems where significant density changes occur as the contents flash on pressure changes. Density changes with pressure in two phase systems can be significant and are frequently also time dependent (requiring a dp/dt term in the calculations) as the system takes time to degas.
3) Pressure is not constant throughout the vessel as head pressure can have a significant impact along with temperature gradients in large vessels (cryogenic storage is notorious for this and has resulted in fatalities when what is on the bottom of the vessel rolls over to the top and flashes to gas (and removes the top of the vessel)). Distillation columns are also an issue for this, if flooded (and ditto on the fatalities).
4) The effects of temperature and pressure in the measurement system (especially on wet leg fills) can also have an impact as these are often outside of the process and can have quite different densities than the fluid in the storage vessel due to ambient temperature effects and ambient temperature compensation may be required for accurate level measurement. Displacer and float type level transmitters and switches have also issues with change of dimensions with pressure and temperature leading to changes in readings with process variables. Filled system and chemical seals are not immune to this effect either (and that assumes the person building the seals knows what they are doing and has degassed the fill fluid and ensured the seal faces are free to flex enough to accommodate the fill fluid volume change (been there…arrrg)). So you need to know your instruments intimately and correct for their specific quirks (and test them thoroughly before trusting them (or the salesman who sold them to you)).
Where accurate dosing is required, mass flow (coriolis) metering and vessel weighing with load cells may be a wise choice.
Where level is important, guided radar usually works well but can have issues with fluids with dielectric constants close to air or vapour (like ethylene (and polyethylene powder) (or steam in supercritical services) ) and gases like hydrogen which tend to remove the insulating coatings off the surface of the sensor by migrating through it and blowing it off on pressure changes or direct hydrogenation of polymer coatings like Teflon (again, been there)). (Again, know your instruments).
Thank You Allan Gibson for sharing valuable knowledge with all of us.
great work, explanation of the transmitter is very elaborate. I have one doubt, does the pressure and temperature of vessel affects the transmitter reading. I have Foxboro 144LD installed in my plant for propylene service and the pressure is around 20Kg/cm2 and temp. is -20DegC, please suggest what density should i use for propylene