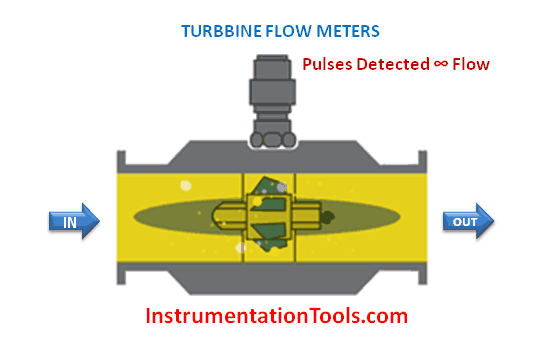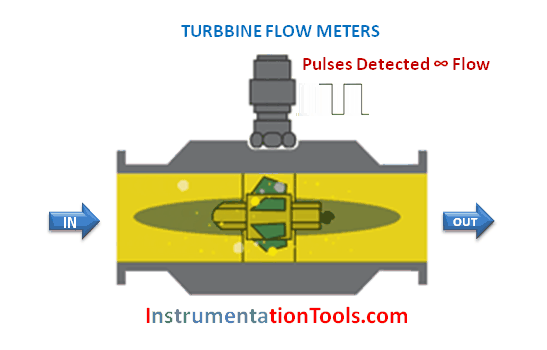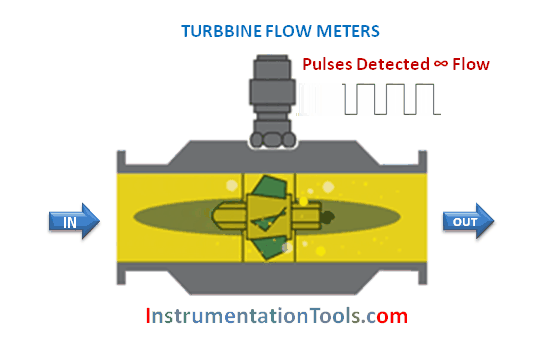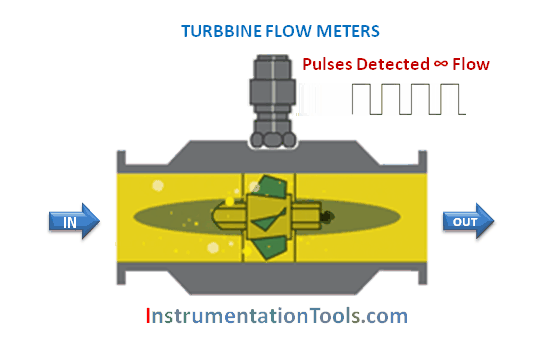
The majority of flowmeter technologies operate on the principle of interpreting fluid flow based on the velocity of the fluid. Magnetic, ultrasonic, turbine, and vortex flowmeters are prime examples, where the sensing elements (of each meter type) respond directly to fluid velocity. Translating fluid velocity into volumetric flow is quite simple, following this equation:
Q = Av
Where,
Q = Volumetric flow rate (e.g. cubic feet per minute)
A = Cross-sectional area of flowmeter throat (e.g. square feet)
v = Average fluid velocity at throat section (e.g. feet per minute)
Positive displacement flowmeters are even more direct than velocity-sensing flowmeters. A positive displacement flowmeter directly measures volumetric flow, counting discrete volumes of fluid as it passes through the meter.
Even pressure-based flowmeters such as orifice plates and venturi tubes are usually calibrated to measure in units of volume over time (e.g. gallons per minute, barrels per hour, cubic feet per second, etc.). For a great many industrial fluid flow applications, measurement in volumetric units makes sense.
This is especially true if the fluid in question is a liquid. Liquids are essentially incompressible: that is, they do not easily yield in volume to applied pressure. This makes volumetric flow measurement relatively simple for liquids: one cubic foot of a liquid at high pressure inside a process vessel will occupy approximately the same volume (≈ 1 ft3) when stored in a barrel at atmospheric pressure.
Gases and vapors, however, easily change volume under the influences of both pressure and temperature. In other words, a gas will yield to an increasing pressure by decreasing in volume as the gas molecules are forced closer together, and it will yield to a decreasing temperature by decreasing in volume as the kinetic energy of the individual molecules is reduced.
This makes volumetric flow measurement more complex for gases than for liquids. One cubic foot of gas at high pressure and temperature inside a process vessel will not occupy one cubic foot under ambient pressure and temperature conditions.
The practical difference between volumetric flow measurement for liquids versus gases is easily seen through an example where we measure the volumetric flow rate before and after a pressure reduction valve:
Flow Measurement
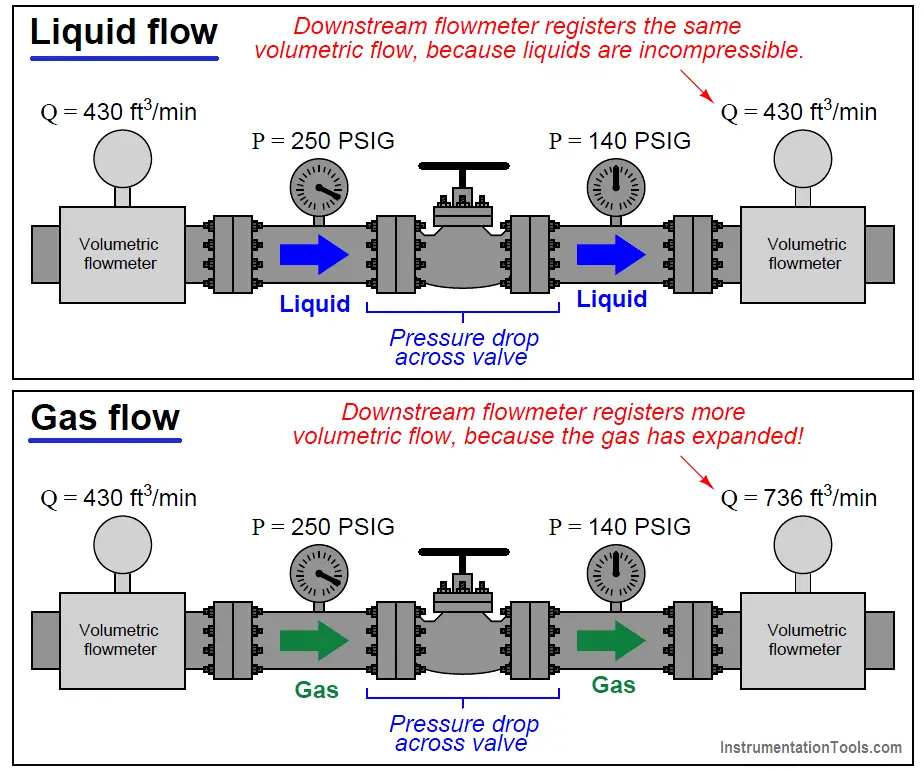
The volumetric flow rate of liquid before and after the pressure-reducing valve is the same, since the volume of a liquid does not depend on the pressure applied to it (i.e. liquids are incompressible). The volumetric flow rate of the gas, however, is significantly greater following the pressure-reducing valve than before, since the reduction in pressure allows the gas to expand (i.e. less pressure means the gas occupies a greater volume).
What this tells us is that volumetric flow measurement for gas is virtually meaningless without accompanying data on pressure and temperature. A flow rate of “430 ft3/min” reported by a flowmeter measuring gas at 250 PSIG means something completely different than the same volumetric flow rate (430 ft3/min) reported at a different line pressure.
One solution to this problem is to dispense with volumetric flow measurement altogether in favor of mass flow measurement, constructing the flowmeter in such a way that the actual mass of the gas molecules is measured as they pass through the instrument.
A more traditional approach to this problem is to specify gas flow in volume units per time, at some agreed-upon (standardized) set of pressure and temperature conditions. This is known as standardized volumetric flow measurement.
Referring to our pictorial example previously shown, imagine if we took a sample of the gas flowing at a line pressure of 250 PSIG and let that sample expand to atmospheric pressure (0 PSIG), measuring its new volume under those new conditions. For the sake of keeping this example simple, we will consider only a change in pressure, but not a change in temperature.
Obviously, one cubic foot of gas at 250 PSIG would expand to a far greater volume than 1 ft3 at atmospheric pressure. This ratio of “standard volume” to “actual volume” (in the pressurized pipe) could then be used to scale the flowmeter’s measurement, so that the flowmeter registers in standard cubic feet per minute, or SCFM:
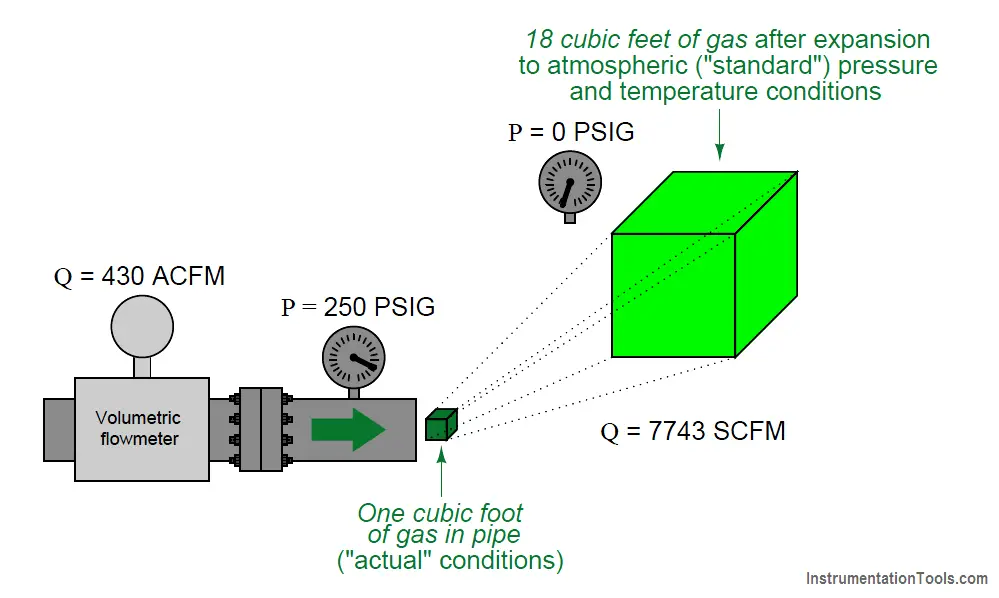
Assuming the same temperature inside the pipe as outside, the expansion ratio for this gas will be approximately 18:1, meaning the “actual” flowing rate of 430 ft3/min is equivalent to approximately 7743 ft3/min of gas flow at “standard” atmospheric conditions.
In order to unambiguously distinguish “actual” volumetric flow rate from “standardized” volumetric flow rate, we commonly preface each unit with a letter “A” or letter “S” (e.g. ACFM and SCFM).
If a volumetric-registering flowmeter is equipped with pressure and temperature sensors, it may automatically scale its own output signal to measure gas flow rate in standard volumetric units. All we need to determine is the mathematical procedure to scale actual conditions to standard conditions for a gas.
To do this, we will refer to the Ideal Gas Law (PV = nRT), which is a fair approximation for most real gases at conditions far from their critical phase-change points. First, we shall write two versions of the Ideal Gas Law, one for the gas under “standard” atmospheric conditions, and one for the gas under “actual” flowing conditions (using the subscripts “S” and “A” to distinguish one set of variables from the other):
PSVS = nRTS
PAVA = nRTA
What we need to do is determine the ratio of standard volume to actual volume ( VS/VA ). To do this, we may divide one equation by the other (Note):
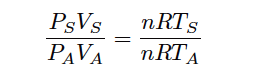
Note : Recall from algebra that we may perform any arithmetic operation we wish to any equation, so long as we apply that operation equally to both sides of the equation. Dividing one equation by another equation obeys this principle, because both sides of the second equation are equal.
In other words, we could divide both sides of the first equation by PAVA (although that would not give us the solution we are looking for), but dividing the left side by PAVA and the right side by nRTA is really doing the same thing, since nRTA is identical in value to PAVA.
Seeing that both the n variables are identical and R is a constant, we may cancel them both from the right-hand side of the equation:
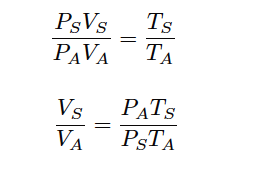
Since we know the definition of volumetric flow (Q) is volume over time ( V/t ), we may divide each V variable by t to convert this into a volumetric flow rate correction ratio (Note):
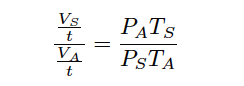
Note : Division by t does not alter the equation at all, since we are essentially multiplying the left-hand side by t t which is multiplication by 1. This is why we did not have to apply t to the right-hand side of the equation.
This leaves us with a ratio of “standardized” volumetric flow (QS) to “actual” volumetric flow (QA), for any known pressures and temperatures, standard to actual:
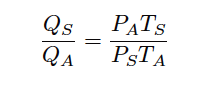
We may apply this to a practical example, assuming flowing conditions of 250 PSIG and 90 degrees Fahrenheit, and “standard” conditions of 0 PSIG and 60 degrees Fahrenheit. It is very important to ensure all values for pressure and temperature are expressed in absolute units (e.g. PSIA and degrees Rankine), which is what the Ideal Gas Law assumes:
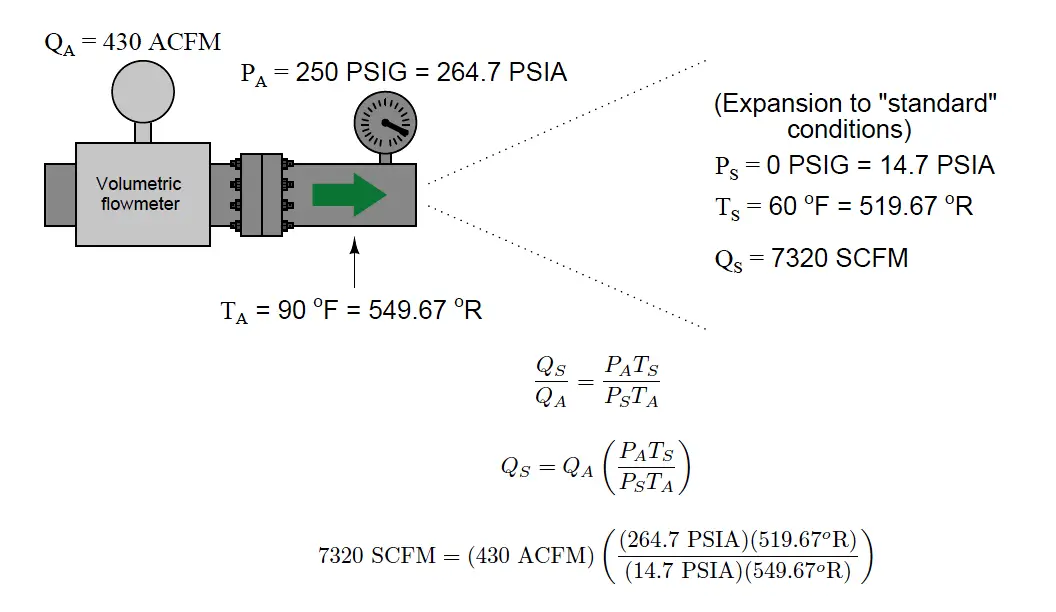
This figure of 7320 SCFM indicates the volumetric flow rate of the gas through the pipe had it been allowed to expand to atmospheric pressure and cool to ambient temperature.
Although we know these are definitely not the same conditions inside the gas pipe, the correction of actual volumetric flow measurement to these imagined conditions allows us to express gas flow rates in an equitable fashion regardless of the process line pressure or temperature.
To phrase this in colloquial terms, standardized volumetric flow figures allow us to compare different process gas flow rates on an “apples to apples” basis, instead of the “apples to oranges” problem we faced earlier where flowmeters would register different volumetric flow values because their line pressures and/or flowing temperatures differed.
With pressure and temperature compensation integrated into volumetric flow meters, we should be able to measure the exact same standardized flow rate at any point in a series gas piping system regardless of pressure or temperature changes:
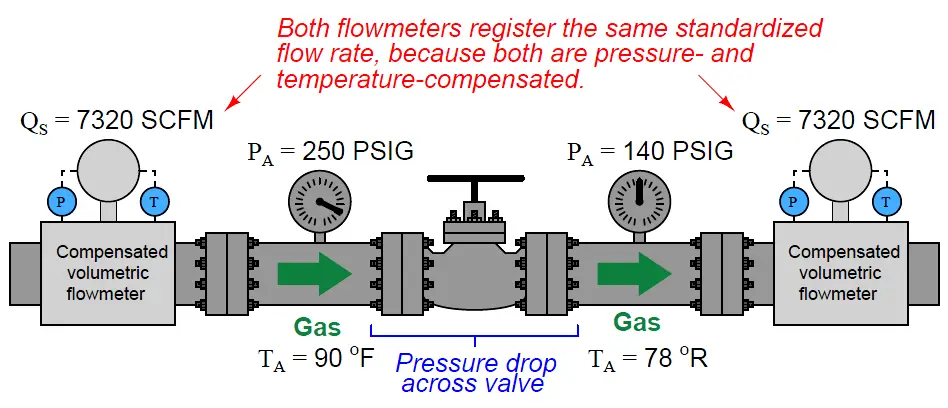
An unfortunate state of affairs is the existence of multiple “standard” conditions of pressure and temperature defined by different organizations. In the previous example, 14.7 PSIA was assumed to be the “standard” atmospheric pressure, and 60 degrees Fahrenheit (519.67 degrees Rankine) was assumed to be the “standard” ambient temperature.
This conforms to the API (American Petroleum Institute) standards in the United States of America, but it does not conform to other standards in America or in Europe. The ASME (American Society of Mechanical Engineers) uses 14.7 PSIA and 68 degrees Fahrenheit (527.67 degrees Rankine) as their “standard” conditions for calculating SCFM.
In Europe, the PNEUROP agency has standardized with the American CAGI (Compressed Air and Gas Institute) organization on 14.5 PSIA and 68 degrees Fahrenheit being the “standard” conditions.
For gas flows containing condensible vapors, the partial pressures of the vapors must be subtracted from the absolute pressures (PA and PS) in order that the correction factor accurately reflects gas behavior alone. A common application of standardized gas flow involving partial pressure correction is found in compressed air systems, where water vapor (relative humidity) is a factor.
Here too, standards differ as to the humidity conditions of “standard” cubic feet. The API and PNEUROP/CAGI standards call for 0% humidity (perfectly dry air) as the “standard,” while the ASME defines 35% relative humidity as “standard” for compressed air calculations.
If you liked this article, then please subscribe to our YouTube Channel for Instrumentation, Electrical, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Flow Proportional to Delta Pressure
- Lobed Impeller Flow Meters
- Flow Measurement Questions
- Rangeability of Flow Sensors
- Multi phase Flow meter Principle











What’s Happening i am new to this, I stumbled upon this I’ve found It positively helpful and it has aided me out loads. I hope to contribute & assist other users like its helped me. Great job.
So, in the Gas flow calculation between Vm = n PSIG and Vb = 0 PSIG algorithm is embedded on Electronic Volume Corrector (EVC) on Meter and Regulating Station Skid for gas custody transfers. (See more on AGA 3, AGA 7, AGA 8 , N – GERG Algorithm