What is conductivity ?
Conductivity indicates the degree of ease with which current can flow through a solution and is represented by the reciprocal of specific resistance.
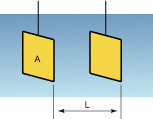
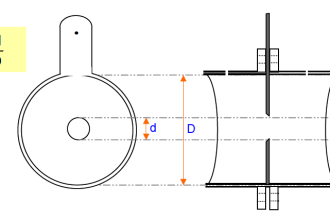
As shown in the following figure, when pole plates of surface area A (cm2) oppose each other in the solution, the electric resistance Rc(Ω) is represented by Rc = r·L/A where r is specific resistance (Ω·cm), which indicates the degree of difficulty for current to flow depending on the solution.
When the conductivity is K (S/cm), K is determined by J/Rc. J is referred to as a cell constant and is a specific value which varies with the shape of the sensor, etc. In addition, if L and A are given because of J = L/A, the conductivity of K is derived by measuring the resistance Rc of the solution.
The unit of conductivity is S/m in SI units. The 1 S/m is equivalent to conductivity S between two electrodes each having an area of 1 m2 separated by a distance of 1 m. Because S/m value does not fit realistic feeling, the conventional μS/cm value is used.Conductivity is also referred to as electrical conductivity or conductance.
Relationship between conductivity and concentration ?
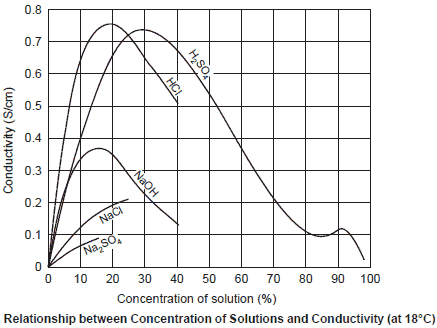
When an electrolytic substance is dissolved in pure water, the conductivity increases in proportion to its concentration so long as the amount dissolved is small.
However, when the concentration of the electrolytic substance is increased beyond a certain level (easy to generate ions), the increase in disassociation (ease of generation of ions) tends to gradually stop.
When the concentration is increased further, the conductivity decreases due to interactions between ions.
The following shows typical relationships between the concentration and conductivity. The actual relationships are not simple. Two maximum values appear in sulfuric acid (H2SO4), etc., and sodium chloride (NaCl) does not dissolve beyond a certain level and its increase in conductivity lessens.
When there is only one kind of substance (NaCl) in water, its concentration can be determined by measuring its conductivity, but it is not possible to know the concentration of each ion from the value obtained by measuring the conductivity of a solution of multiple kinds of substances.
What is temperature compensation (reference temperature conversion) of a test solution ?
The conductivity of a solution greatly changes depending on its temperature. Generally, the conductivity of a solution increases with temperature, but the degree of increase varies with the type and concentration of ions.
Therefore, it is necessary to keep the solution temperature constant when decisions are made using values of conductivity. Because it is difficult in practice to keep the solution temperature constant, the temperature is measured and converted into a reference temperature.
For industrial measurements, a temperature measurement device is built into the operation circuit for automatic conversion into the reference temperature.
Temperature compensation is generally performed based on the temperature coefficient of dilute NaCl solution, but when the NaCl dilution solution is significantly different from the temperature coefficient, it is necessary to set the coefficient manually.
What kind of method does the measurement of the conductivity have ?
The two most common methods used to measure conductivity are:
(1) Electrode; and
(2) Inductive.
There are two types of electrode method for measuring conductivity: the 2-electrode method and the 4-electrode method, which are hardly influenced by dirt and polarization.
The inductive method is suitable for measuring a highly concentrated acid or alkaline solution, but is not suitable for measuring a low-conductivity aqueous solution such as pure water.
Calibration of conductivity sensors ?
There is no standard or national organization that specifies a standard liquid and device for contacting conductivity analyzers. Generally, these analyzers are calibrated using a calibration solution prepared.
For this reason, the value of the conductivity sensor is assigned based on the absolute value of the calibration solution to be used. Because the sensor of each contacting conductivity analyzer does not deteriorate significantly with time unlike the pH meter glass electrode, it rarely needs to be calibrated.
As for the calibration solutions, what are used ?
The calibration solutions for contacting conductivity analyzers generally include sodium chloride (NaCl) and potassium chloride (KCl) which can be prepared in the laboratory.
How should calibration solutions be stored, and for how long ?
The prepared calibration solution is hermetically sealed in a bottle made of high-quality hard glass or polyethylene.
Because the conductivity of a prepared calibration solution changes during long-term storage, it is important to check whether the conductivity of calibration solution stored for a long time is the same as that of newly prepared calibration solution before it is used.
In addition, calibration solution must not be reused once it has been opened and left exposed to the air.
Accuracy of conductivity meters ?
The accuracy of conductivity meters is not defined (but that of pH meters is defined). Unlike pH meters, there is no national organization or standard that specifies the accuracy of the standard solution and device itself.
At present, all manufacturers define the accuracy of each conductivity meter in terms of the repeatability and linearity in the pseudo input resistance of the converter itself, rather than a combination of converter (or transmitter) and sensor.
Relationship between conductivity and resistivity
Resistivity (Ω·cm) is represented as the reciprocal of conductivity (S/cm) and is also referred to as specific resistance. In the water treatment industry, water with a resistance of 1 MΩ·cm or more (conductivity of 1 μS/cm or less) is represented by its resistivity.
What is the difference between a conductivity analyzer and an inductive conductivity analyzer?How should they be used differently?
It is recommended that the conductivity analyzer is used for deionized water or purified water with low conductivity.
The inductive conductivity analyzer is used for measuring dirty water or acid/alkaline solution with high conductivity. Indication for normal measurement is around 100 μS/cm.
Is the conductivity meter measuring conductivity or conductance?
Conductivity is the measurement of the electrolytes in a solution. It is defined as the conductance in a given volume of sample. Conductance is the ability of the solution to conduct electric current.
Conductivity = Conductance x Probes cell constant (K)
Conductivity = Electrical Current/Voltage x Distance/Area
How does temperature affect conductivity readings?
The effect of temperature on conductivity readings depends on the solution being measured. The effect is greatest in low ionic strength (low conductivity) solutions.
A general rule to follow is there will be a 2% change (increase)/degree C. This rule can be followed for most aqueous solutions, however if you require a high degree of accuracy, you should consult a chart for the particular solution you are measuring.
Can conductivity be measured in aqueous solutions only?
No, all substances possess some conductive properties. Generally organic compounds (such as benzene, alcohols, and petroleum products) have very low conductivities, while metals have very high conductivities. Measuring the conductivity of highly flammable liquids is very risky.
How are conductivity and TDS related?
Salts, minerals, and even dissolved gases contribute uniformly to the conductivity of a solution. This means that the conductivity can be used as an indicator of the amount of dissolved materials in a solution.
TDS can be used fairly accurately when determining the concentration of a single salt, such as NaCl, but error can arise when trying to compare two different types of solutions. It is necessary to calibrate the meter using the same dissolved materials that are in the test solution.
What is the difference between conductivity and salinity?
The probe is the same for conductivity and salinity, but for salinity readings a correction factor is applied to the conductivity value.
The correction factor takes the conductivity reading and converts it to ppm of NaCl.
What is a cell constant K and why are there probes with different values of K?
The cell constant, K, is equal to the distance in cm between the probe’s electrodes divided by the surface area of the electrodes in cm2.
For solutions with low conductivities the electrodes can be placed closer together or made larger so that the cell constant is less than one. This has the effect of raising the conductance to produce a value more easily interpreted by the meter.
The reverse also applies, in high conductivity solutions, the electrodes are placed farther apart or made smaller to reduce the conductance of the sample.
By using the appropriate probe, K=0.1 for low conductivity solutions, K=1 for normal solutions and K=10 for high conductivity solutions, accurate measurements across the full range of conductivity values can be made.
How do I find the correct temperature coefficient when not working with water?
For water, the correction factor is set at a default value of 1.91% per degree C. Check the conductivity of the sample at 25°C, then using the same sample, find the conductivity at another temperature to see what the percent change is. This will give you the temperature correction factor.
How should conductivity probes be cleaned?
Clean cells with mild liquid detergent and/or dilute nitric acid (1% wt) by dipping or filling the cell with solution and agitating for 2 to 3 minutes. Dilute HCl (hydrochloric acid) or H2SO4 (sulfuric acid) may also be used.
When stronger cleaning is needed, try concentrated HCl mixed into 50% isopropanol (rubbing alcohol). Rinse the cell several times with distilled or deionised water and recalibrate before use.
How should conductivity probes be stored?
Rinse it in distilled/deionised water when you are finished using it. You can store your electrode either wet or dry. If it is stored dry you will need to recondition the electrode before use.
How should a meter for TDS be calibrated if the dissolved solids are not the same as those in the calibration solution?
Making your own standard will yield the most accurate results. This is done by formulating a mixture of salts in relative proportions to those that simulate the solution being tested, then dissolving the mixture into distilled water.
This should be done according to the following formula:
1 mg salt mixture/litre of distilled water = 1 ppm TDS,
or,
X ppm TDS = X mg of salts + one litre of distilled water.
Remember that “X mg of salts” is the number of milligrams of the mixture of salts which simulate your test solution, not X milligrams of each salt in the mixture. An appropriate value for “X” is determined by the following rule:
Choose a ppm value for a calibration solution which is as close as possible to the expected ppm values of the test solutions. If the ppm content of the test solution is expected to vary a great deal, it is best to choose a ppm value for the calibration solution in the upper 1/3 of the expected TDS measurement range.
Note: Above mentioned Conductivity Analyzer Parameters may change from vendor to vendor
Also Read: pH Analyzers Interview Questions & Answers








Conductivity working principle