Gas chromatography is a very important process in the chemical industry. This process is also termed a landmark innovation in the instrumentation part.
In this post, we will learn the concept of gas chromatography (GC).
What is Gas Chromatography?
Chromatography means to separate something basically. If we go deeper, it means separating organic compounds and molecules present inside a media (liquid or gas) in chemical terms.
Basically, there are many requirements where it is needed to analyze a chemical to detect what is present inside it. This itself can show us much useful this process is. You can easily then identify the molecules and organics present inside it.
Now, gas chromatography is a term used because here, gas is used as a medium to separate the compounds. Gas is a very fast-acting medium and it helps in transporting the molecules out of the chromatographer easily.
The gas used is such that it does not react with the molecules; otherwise, it is of no use, as it will completely misalign the compound mixture and give a totally abnormal result. The gas used is an inert one, usually nitrogen, argon, hydrogen, or helium.
The gas used depends on the product used (here product means the media which has molecules to be separated), analysis time, molecular weight, flow rate required, thermal conductivity, and temperature sensitivity.
Working Principle of Gas Chromatography
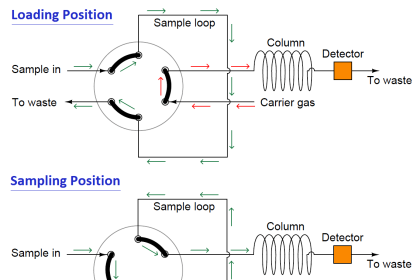
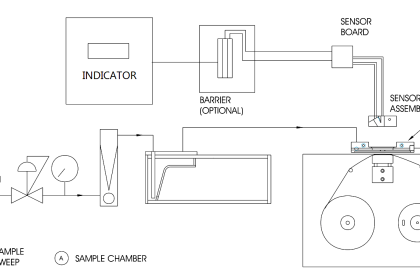
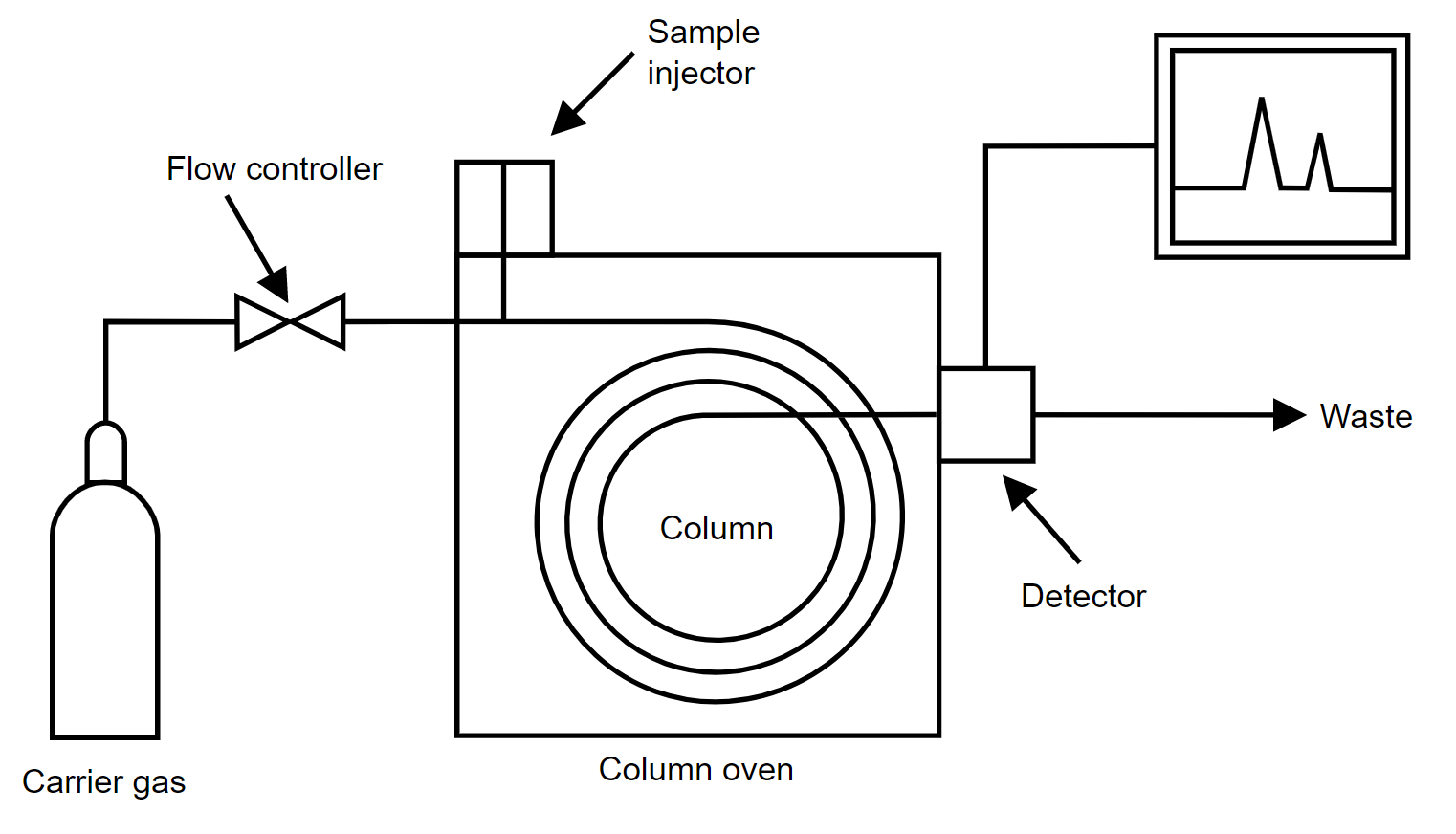
The gas chromatography process consists of five main components – mobile phase (gas carrier we discussed earlier), sample port (where the product will be fed), stationary phase (liquid or gas with a high boiling point, for aiding the separation process), detector (for detecting the molecules) and computer (for giving final results by analyzing the molecules).
The process starts with the sample port and mobile phase. The mobile phase is the name given because it is the one that moves and changes the state of the sample product given. The gas must be pure and contaminant free, to avoid any unwanted reactions.
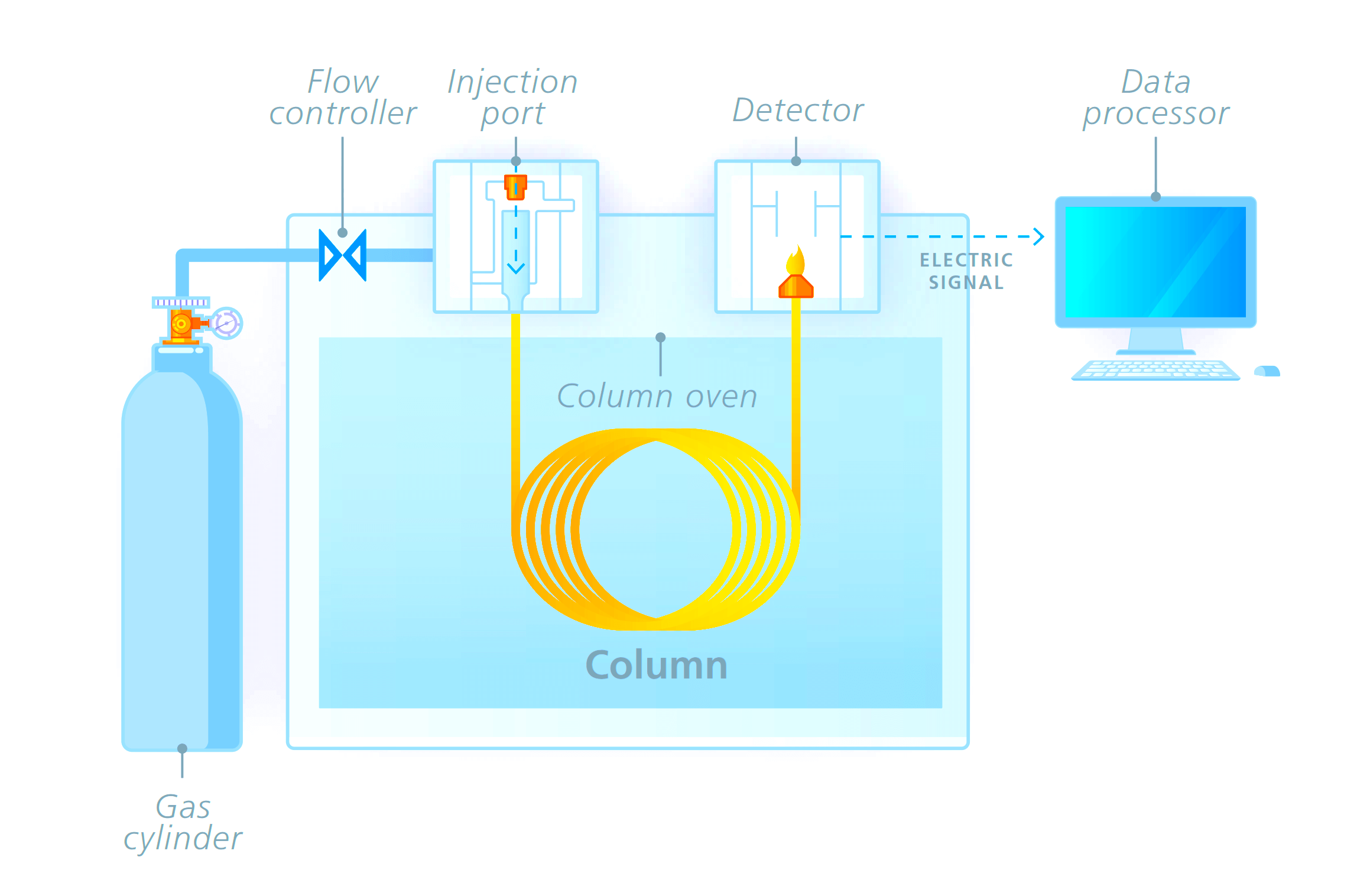
The gas used is preheated already. It cannot be cold; otherwise, it will not be able to give the required effect to the sample. The sample port is used to provide sample products to the chromatographer.
Injection techniques are used to insert the sample. It is delivered inside in microliters. Both the mobile phase gas and sample product combines in a vaporization chamber, which is maintained above the lowest boiling point.
Due to such a large amount of heat from the gas and inside the chamber, the sample product is vaporized automatically. If the product is already in gaseous form, then its boiling point changes due to the amount of heat to which it is subjected. This makes it easier for the gas to transport the product inside the chamber columns with stationary phase media.
The stationary phase consists of another liquid media. It is used to selectively absorb or retain the sample molecules. Its temperature is maintained always below the high boiling point. This helps it to retain its original state.
The stationary phase is present inside a column oven, which maintains a constant temperature within the boiling point. This helps in properly executing the isothermal operation.
Now, when the sample and mobile phase gas mixture in vaporized form enters the column filled with the stationary phase, the molecules start to mobilize. With this, the compounds present in the mixture which have the lowest boiling point start to run fast away to the detector.
Due to this, these compounds reach the detector first; and it helps in analyzing one set of compounds. The molecules with a medium boiling point reach the detector a little slowly.
In this sequence, similar types of molecules with a boiling point reach in groups to the detector in ascending order (from low to high). This concludes that the highest boiling point will reach the detector late.
The most common detectors used are flame ionization detector, thermal conductivity detector, electron capture detector, mass spectrometer, and atomic emission.
Finally, the data is sent to a computer system that performs the operation of identification, injection sequence analysis, temperature monitoring, flow rate monitoring, and wavelength detection.
In this way, we understand the general concept regarding gas chromatography.
Download the below document to understand more about GC.
| Title: | Basics & Fundamentals Gas Chromatography |
| Author: | Shimadzu |
| Format: | |
| Size: | 739 KB |
| Pages: | 21 |
| Download: | Click Here |
If you liked this article, then please subscribe to our YouTube Channel for Electrical, Electronics, Instrumentation, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Infrared Flue Gas Analyzer
- Moisture Analyzer Problems
- Silica Analyzer Troubleshooting
- Selection Criteria of pH Analyzers
- Oxygen Measurement in Flue Gas