In this article, we will learn the measurement of oxygen (O2) in flue gas, the types of principles, and their working principles.
What is Flue Gas?
Flue gas is the exhaust gas from combustion processes within the power plants, flue gases come in many different forms that vents to the atmosphere through the flue duct, and chimney opening that enables the gases to travel from the source to the local atmosphere.
What are the Flue Gas Constituents?
The constituents within the flue gas will vary from plant to plant. These include nitrogen, carbon dioxide, oxygen, carbon monoxide, hydrogen fluoride, sulphur oxides, ammonia, and various volatile organic compounds (VOCs) and water vapour, as well as solid particles arising from incomplete combustion processes.
What are the Chemicals used in the Orsat Apparatus?
The chemicals used in the Orsat apparatus are carbon dioxide, oxygen, and carbon monoxide.
Oxygen is the most important element for all of us. Air is used to meet the requirement of oxygen. The atmospheric air contains only 21 % of oxygen by volume.
The mechanical draught systems used as auxiliaries to the boiler serve the oxygen in terms of air,
But the oxygen requirement in the furnace depends upon the type of fuel which we are using.
For proper burning of fuel in the boiler furnace, oxygen is the most essential element required and must have an adequate or extra supply of oxygen or air for complete combustion this is achieved by stoichiometric ratio.
The stoichiometric ratio is the theoretical fuel-to-air ratio required for combustion in a furnace.
The mixture is said to be fuel-rich when the air supplied is less than the fuel supply. And the mixture is said to be air rich when the air supplied is more than the fuel.
The percentage of oxygen present in flue gas determines the thermal efficiency of the boiler. To achieve boiler thermal efficiency the fuel-to-air ratio must be adequate.
Measurement of Oxygen
The measurement of oxygen in the flue gas indicates the zone in which the boiler is operating. If the percentage of oxygen is more than the boiler then it is said to be operating at a higher excess air region.
The excess air for combustion in the furnace is obtained by the proper mixture of air and fuel ratio.
The primary standard for volumetric analysis for combustion products is done by the Orsat apparatus which works on the principle of chemical absorption.
The unit of measurement of the flue gas is expressed in parts per million (PPM) or percentage.
The two principles involved in oxygen analysis are.
- Using the paramagnetic property of oxygen.
- Using zirconium oxide probes.
Paramagnetic Oxygen Analyzer
Among industrial combustible gases like O2, CO2, H2, CO, NO, and NO2, the only oxygen is highly paramagnetic.
As the temperature of gases increases the paramagnetic property of oxygen decreases.
The poles of a permanent magnet are extended into a test cell made of a non-magnetic material. A platinum resistance wire can be electrically heated to about 250 to 320 Degrees Celsius and is mounted in the zone of maximum field strength.
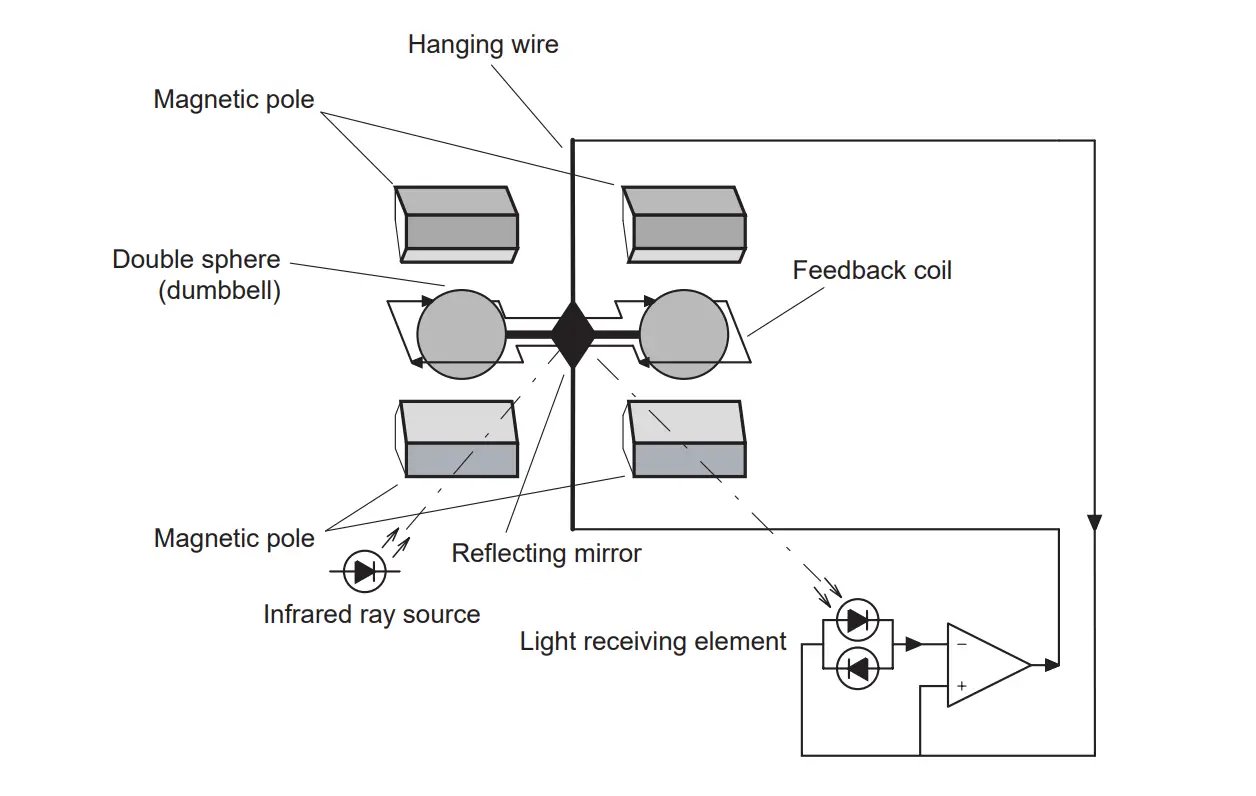
Circulation of the flue gas is produced due to thermal up-drift. The magnet at first exerts a strong attraction upon the oxygen present.
The active oxygen particles are replaced instead of the latter, as it loses their paramagnetic property during heating.
The resultant gas circulation cools the platinum wire and causes unbalanced voltage in the bridge which is a measure of oxygen percentage in the flue gas.
The basic wheatstone bridge is used for the measurement of oxygen percentage in flue gas using the principle of paramagnetism.
The oxygen is influenced by the magnetic field and is forced to flow through the heated platinum wire placed in the magnetic field. And both the arms of the Wheatstone bridge are exposed to the magnetic field to increase or double its sensitivity.
The other two arms have dummy pole shoes to give a reference for the effect of the thermal up-drift while retaining geometric symmetry.
Zirconium Oxygen Analyser
In a paramagnetic oxygen analyzer, the flue gas analysis is done by extracting the sample, filtering it, and cooled before passing it to the probe.
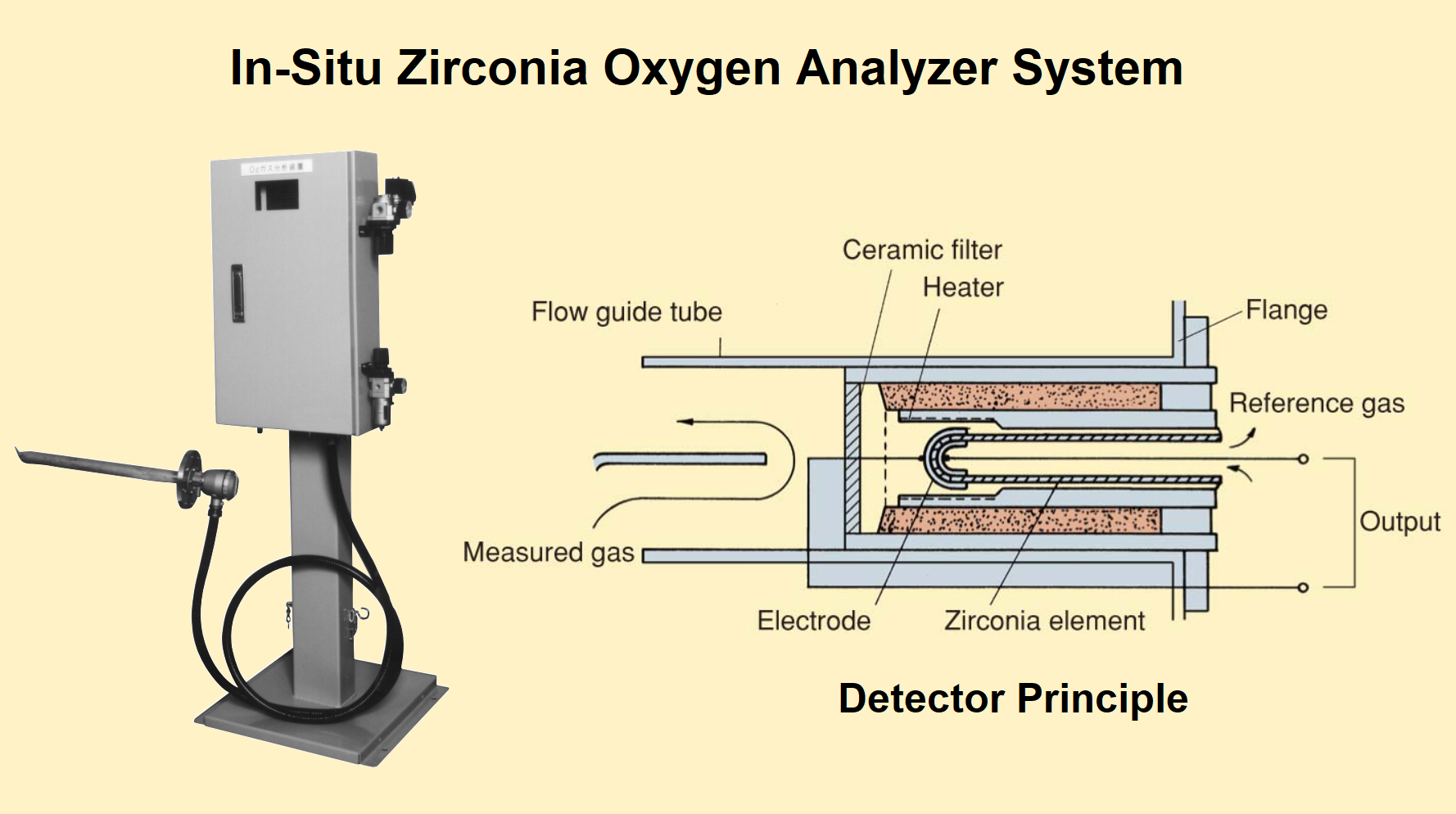
But the frequent maintenance is necessary to prevent in-situ sampling system blockage. The probe must be calibrated using specialized skills to keep it in operation.
The solid-state zirconia gas analyzer is designed using rugged and non-sampling techniques in such a way that it should withstand the flue gas environment around the rugged and son-sampling
The zirconium oxide (ZrO2) is the ceramic material that acts as a permeable membrane to oxygen when it is heated. In other words, the zirconium oxide ceramic sensing element is a closed-end tube that when hot (= 700°C) conducts oxygen ions. The platinum coating on the inside and outside of the tube serves as electrodes.
Depending upon the ratio of partial pressure the voltage signal in the sensor is developed when two electrodes are in contact with gases having different levels of partial pressure and the following equation satisfies the condition
E = RT/4F * log (P1/P2) + C
Where
E = Output voltage
R = Universal Gas constant
P1 = Partial pressure of oxygen on the Reference gas side. Normally air is used
P2 = Partial pressure of oxygen in the flue gas.
C = Cell constant
T= Absolute temperature of the cell
F = Faraday’s constant.
The platinum electrodes, and the reference gas as a source of air are used to build the zirconia analyzer probe.
The partial pressure of oxygen on the reference side of the cell is known and hence the oxygen content of the flue gas is represented by the E.M.F produced by the cell.
To protect the zirconia analyzer probe the porous filter is used. The most common form of the zirconia analyzer comprises a probe-mounted zirconia element inserted into the flue duet.
In the boiler furnace, the probe tip is heated to a higher temperature of about 830 Degrees Celsius. The electrical signal from the probe is received by a microprocessor-based electronic unit to calculate the O2 concentration.
Advantages
- Quick response.
- Stable measurement for a long time
- Due to the absence of the sampling portion, no maintenance or little maintenance is required.