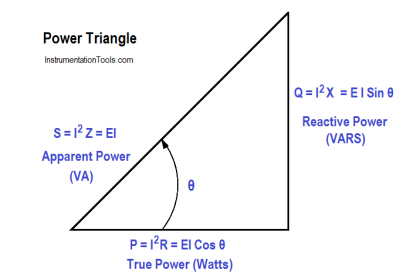
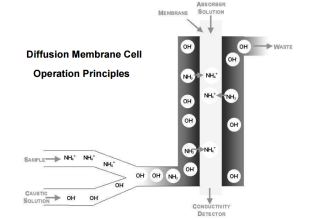
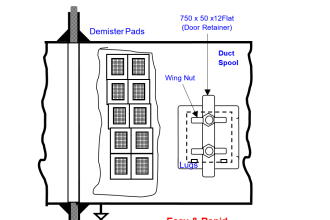
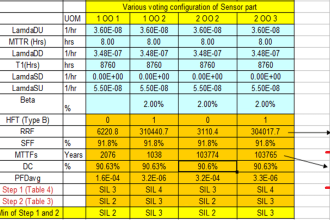
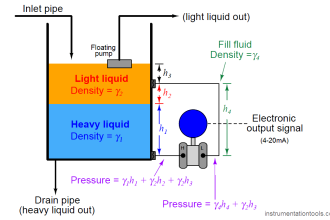
The lead-acid battery is the most common type of battery in use today. There are other types of storage batteries, each having certain advantages.
Types of batteries are :
- Carbon-zinc cell
- Alkaline cell
- Nickel-cadmium cell
- Edison cell
- Mercury cell
Wet and Dry Cells
Wet and dry cells are classified by the type of electrolyte the battery uses. The electrolyte of a cell may be a liquid or a paste. If the electrolyte is a paste, the cell is referred to as a dry cell. If the electrolyte is a solution, the cell is called a wet cell.
Carbon-Zinc Cell
The carbon-zinc cell is one of the oldest and most widely used types of dry cells. The carbon in the battery is in the form of a rod in the center of the cell which acts as the positive terminal. The case is made from zinc and acts as the negative electrode. The electrolyte for this type of cell is a chemical paste-like mixture which is housed between the carbon electrode and the zinc case. The cell is then sealed to prevent any of the liquid in the paste from evaporating.
The advantage of a carbon-zinc battery is that it is durable and very inexpensive to produce. The cell voltage for this type of cell is about 1.5 volts.
Alkaline Cell
The alkaline cell is so called because it has an alkaline electrolyte of potassium hydroxide. The negative electrode is made from zinc, and the positive electrode is made of manganese dioxide. The typical alkaline cell generates 1.5 volts. The alkaline cell has the advantage of an extended life over that of a carbon-zinc cell of the same size; however, it is usually more expensive.
Nickel-Cadmium Cell
The nickel-cadmium cell is a secondary cell, and the electrolyte is potassium hydroxide. The negative electrode is made of nickel hydroxide, and the positive electrode is made of cadmium hydroxide. The nominal voltage of a nickel-cadmium cell is 1.25 volts.
The nickel-cadmium battery has the advantage of being a dry cell that is a true storage battery with a reversible chemical reaction (i.e., it can be recharged). The nickel-cadmium battery is a rugged, dependable battery. It gives dependable service under extreme conditions of temperature, shock, and vibration. Due to its dependability, it is ideally suited for use in portable communications equipment.
Edison Cell
In an edison cell the positive plate consists of nickel and nickel hydrate, and the negative plate is made of iron. The electrolyte is an alkaline. Typical voltage output is 1.4 volts, and it should be recharged when it reaches 1.0 volts. The edison cell has the advantage of being a lighter and more rugged secondary cell than a lead-acid storage battery.
Mercury Cell
Mercury cells come in two types; one is a flat cell that is shaped like a button, while the other is a cylindrical cell that looks like a regular flashlight battery. Each cell produces about 1.35 volts. These cells are very rugged and have a relatively long shelf life. The mercury cell has the advantage of maintaining a fairly constant output under varying load conditions. For this reason, they are used in products such as electric watches, hearing aids, cameras, and test instruments.
Battery Types Summary
- If the electrolyte is a paste, the cell is referred to as a dry cell. If the electrolyte is a solution, the cell is called a wet cell.
- The advantage of a carbon-zinc battery is that it is durable and very inexpensive to produce.
- The alkaline cell has the advantage of an extended life over that of a carbon-zinc cell of the same size.
- The nickel-cadmium battery has the advantage of being a dry cell that is a true storage battery with a reversible chemical reaction.
- The edison cell has the advantage of being a lighter and more rugged secondary cell than a lead-acid storage battery.
- The mercury cell has the advantage of maintaining a fairly constant output under varying load conditions.