A battery converts chemical energy to electrical energy. This conversion enables electrical power to be stored.
The purpose of a battery is to store chemical energy and to convert this chemical energy into electrical energy when the need arises.
As described in previous article, a chemical cell (or voltaic cell) consists of two electrodes of different types of metals or metallic compounds and an electrolyte solution which is capable of conducting an electric current.
A good example of a voltaic cell is one that contains zinc and copper electrodes. The zinc electrode contains an abundance of negatively charged atoms, and the copper electrode contains an abundance of positively charged atoms. When these electrodes are immersed in an electrolyte, chemical action begins.
The zinc electrode will accumulate a much larger negative charge because it dissolves into the electrolyte. The atoms, which leave the zinc electrode, are positively charged and are attracted by the negatively charged ions of the electrolyte; the atoms repel the positively charged ions of the electrolyte toward the copper electrode (Figure 2).
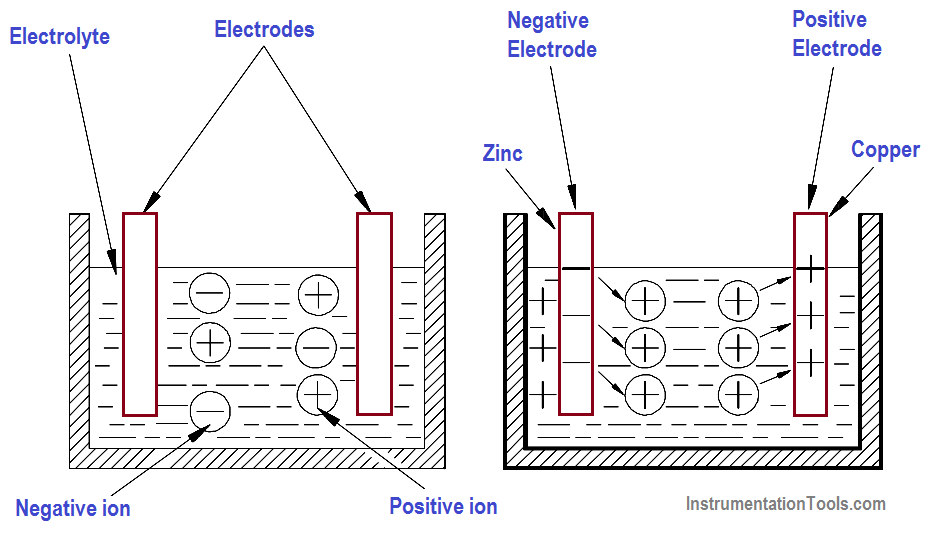
Figure 2 Basic Chemical Production of Electrical Power
This action causes electrons to be removed from the copper electrode, leaving it with an excess of positive charge. If a load is connected across the electrodes, the forces of attraction and repulsion will cause the free electrons in the negative zinc electrode to move through the connecting wire and load, and toward the positive copper electrode (Figure 3).
The potential difference that results allows the cell to function as a source of applied voltage.
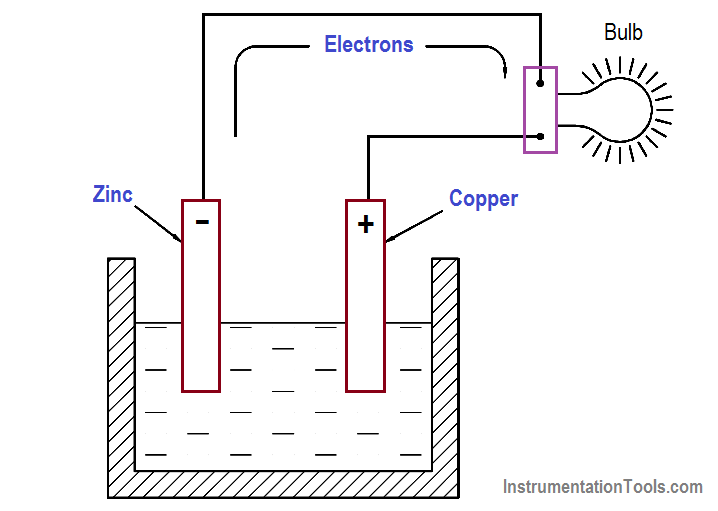
Figure 3 Electron Flow Through a Battery