A battery consists of two or more chemical cells connected in series. The combination of materials within a battery is used for the purpose of converting chemical energy into electrical energy. To understand how a battery works, we must first discuss the chemical cell. The chemical cell is composed of two electrodes made of different types of metal or metallic compounds which are immersed in an electrolyte solution.
The chemical actions which result are complicated, and they vary with the type of material used in cell construction. Some knowledge of the basic action of a simple cell will be helpful in understanding the operation of a chemical cell in general.
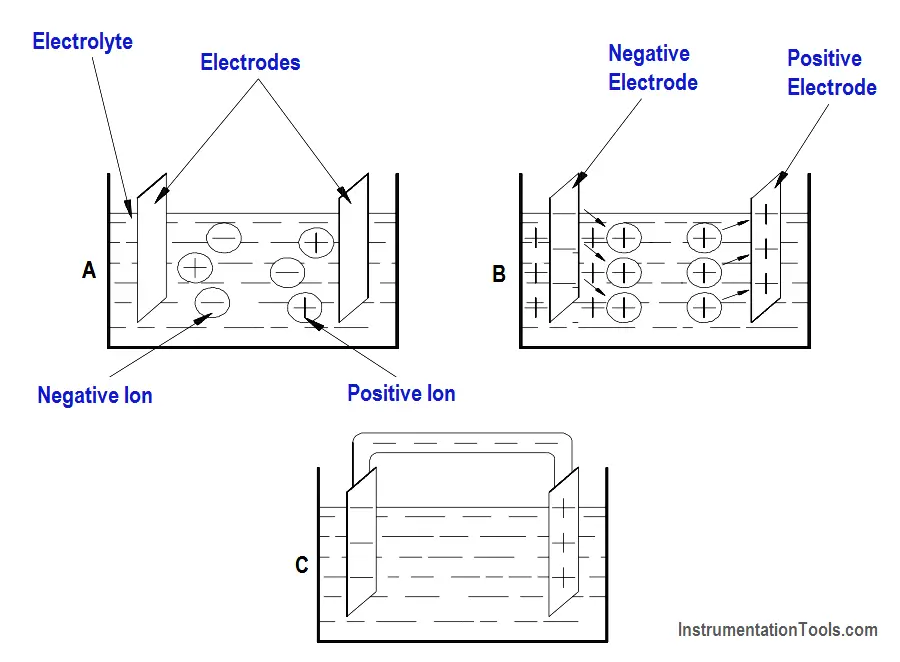
In the cell, electrolyte ionizes to produce positive and negative ions (Figure 1, Part A). Simultaneously, chemical action causes the atoms within one of the electrodes to ionize.
Figure 1 Basic Chemical Battery
Due to this action, electrons are deposited on the electrode, and positive ions from the electrode pass into the electrolyte solution (Part B). This causes a negative charge on the electrode and leaves a positive charge in the area near the electrode (Part C).
The positive ions, which were produced by ionization of the electrolyte, are repelled to the other electrode. At this electrode, these ions will combine with the electrons. Because this action causes removal of electrons from the electrode, it becomes positively charged.