Imagine a major marathon race, where hundreds of runners gather in one place to compete. When the starting gun is fired, all the runners begin running the race, starting from the same location (the starting line) at the same time. As the race progresses, the faster runners distance themselves from the slower runners, resulting in a dispersion of runners along the race course over time.
Now imagine a marathon race where certain runners share the exact same running speeds. Suppose a group of runners in this marathon all run at exactly 8 miles per hour (MPH), while another group of runners in the race run a bit slower at exactly 6 miles per hour, and another group of runners plod along at exactly 5 miles per hour. What would happen to these three groups of runners over time, supposing they all begin the race at the same location and at the exact same time?
As you can probably imagine, the runners within each speed group will stay with each other throughout the race, with the three groups becoming further spread apart over time.
The first of these three groups to cross the finish line will be the 8 MPH runners, followed by the 6 MPH runners a bit later, and then followed by the 5 MPH runners after that.
To an observer at the very start of the race, it would be difficult to tell exactly how many 6 MPH runners there were in the crowd, but to an observer at the finish line with a stop watch, it would be very easy to tell how many 6 MPH runners competed in the race, by counting how many runners crossed the finish line as a distinct group at the exact time corresponding to a speed of 6 MPH.
Now imagine a mixture of chemicals in a fluid state traveling through a very small-diameter “capillary” tube filled with an inert, porous material such as sand. Some of those fluid molecules will progress more easily down the length of the tube than others, with similar molecules sharing similar propagation speeds.
Chromatography
Thus, a small sample of that chemical mixture injected into such a capillary tube, and carried along the tube by a continuous flow of solvent (gas or liquid), will tend to separate into its constituent components (called species) over time just like the crowd of marathon runners separate over time according to running speed.
Slower-moving molecules will experience greater retention time inside the capillary tube, while faster-moving molecules experience less.
Also See : Chromatography Working Animation
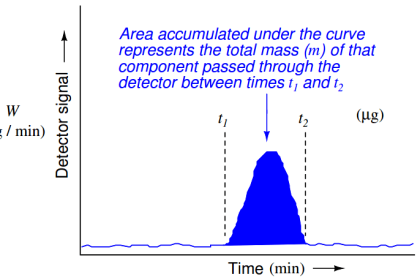
A detector placed at the outlet of the capillary tube, configured to detect any chemical different from the solvent, will indicate the different species exiting the tube at different times.
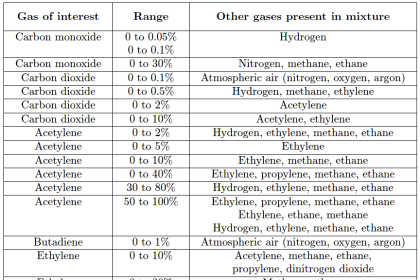
If the retention time of each chemical species is known from prior tests, this device may be used to identify the composition of the original chemical mix (and even how much of each species was present in the injected sample) based solely on the time delay of each species exiting the column.
More importantly, this one device will be able to identify and quantify a great many chemical compounds present in the original sample, a feat unmatched by most analytical technologies.
This is the essence of chromatography: the technique of chemical separation by time-delayed travel down the length of a stationary medium (called a column).
In chromatography, the chemical solution traveling down the column is called the mobile phase, while the solid and/or liquid substance residing within the column is called the stationary phase. Chromatography was first applied to chemical analysis by a Russian botanist named Mikhail Tswett, who was interested in separating mixtures of plant pigments.
The colorful bands left behind in the stationary phase by the separated pigments gave rise to the name “chromatography,” which literally means “color writing.”
Modern chemists often apply chromatographic techniques in the laboratory to purify chemical samples, and/or to measure the concentrations of different chemical substances within mixtures.
Some of these techniques are manual (such as in the case of thin-layer chromatography, where liquid solvents carry liquid chemical mixtures along a flat plate covered with an inert coating such as alumina, and the positions of the chemical drops after time distinguishes one chemical species from another).
Other techniques are automated, with machines called chromatographs performing the timed analysis of chemical travel through tightly-packed tubular columns. The main focus of this section will be automated chromatography, as is used for continuous process analysis.
Also Read : Chromatography Questions & Answers






Nice explanation. Thanks a lot. All
Your articles are enough for Instrumentation Graduates instead of studying 4 years without understanding.