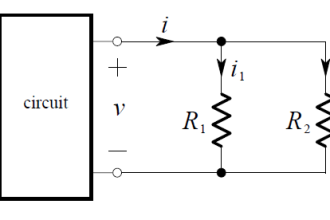
Thermal conductivity detectors work on the principle of heat transfer by convection (gas cooling). Here, the assumption is that sample compounds will have different thermal properties than the carrier gas. Recall the dependence of a thermal mass flowmeter’s calibration on the specific heat value of the gas being measured. This dependence upon specific heat meant that we needed to know the specific heat value of the gas whose flow we intend to measure, or else the flowmeter’s calibration would be in jeopardy.
Here, in the context of chromatograph detectors, we exploit the impact specific heat value has on thermal convection, using this principle to detect compositional change for a constant-flow gas rate. The temperature change of a heated RTD or thermistor caused by exposure to a gas mixture with changing specific heat value indicates when a new sample species exits the chromatograph column.
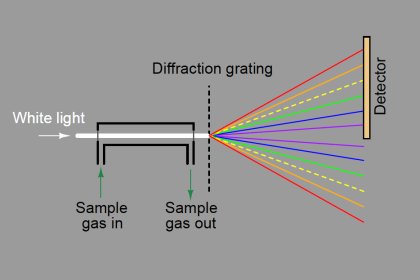
A simplified diagram of a TCD is shown here, with pure carrier gas cooling two of the selfheated thermal sensors and sample gas (mixed with carrier gas, coming off the end of the column) cooling the other two self-heated sensors. Differences in thermal conductivity between gas exiting the column versus pure carrier gas will cause the bridge circuit to unbalance, generating a voltage signal at the output of the operational amplifier circuit:
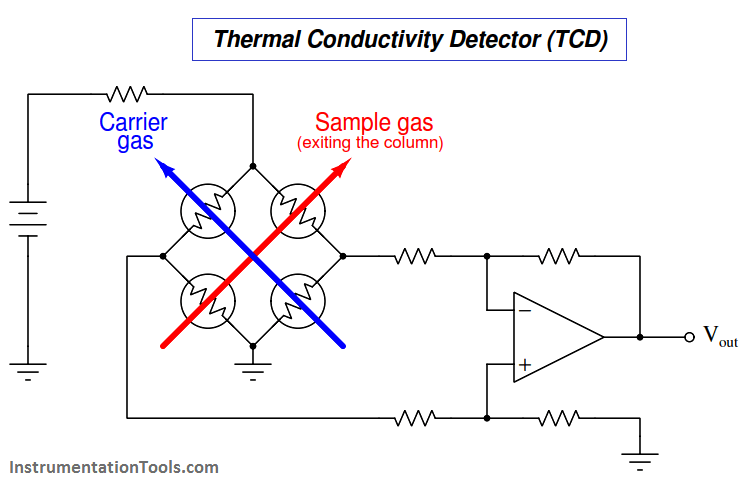
This type of chromatograph detector works best, of course, when the carrier gas has a significantly different specific heat value than any of the sample compounds. For this reason, hydrogen or helium (both gases having very high specific heat values compared to other gases) are the preferred carrier gases for chromatographs using thermal conductivity detectors.
Also Read : GC Principle