In this article, we will discuss about the measurement of POL, pH, Laurentz Polarimeter, and BRIX in the Sugar Industry.
Contents
Pol Measurement
- In the sugar industry pol measurement of cane juice is a quantitative estimation of optically active materials present in cane juice.
- The solution of sucrose and other components has the power to rotate the plane of polarization.
- The angle through which the plane of polarization is rotated can be measured and analyzed by suitable optical instruments and the analysis of sugars by optical methods employs the measurement of this rotation.
- The amount of rotation depends upon the concentration the length of the cell, the wavelength of light, and the temperature of the solution.
- By standardizing the conditions as fixed length of the cell, the temperature, weight, and volume light source the rotation becomes the function of the concentration of the sugar in the solution.
The amount of optical rotation depends upon:
- Crystal Thickness
- Density of Sugar solution.
- Concentration of the solution.
- Temperature of the solution
- Wavelength of the light employed
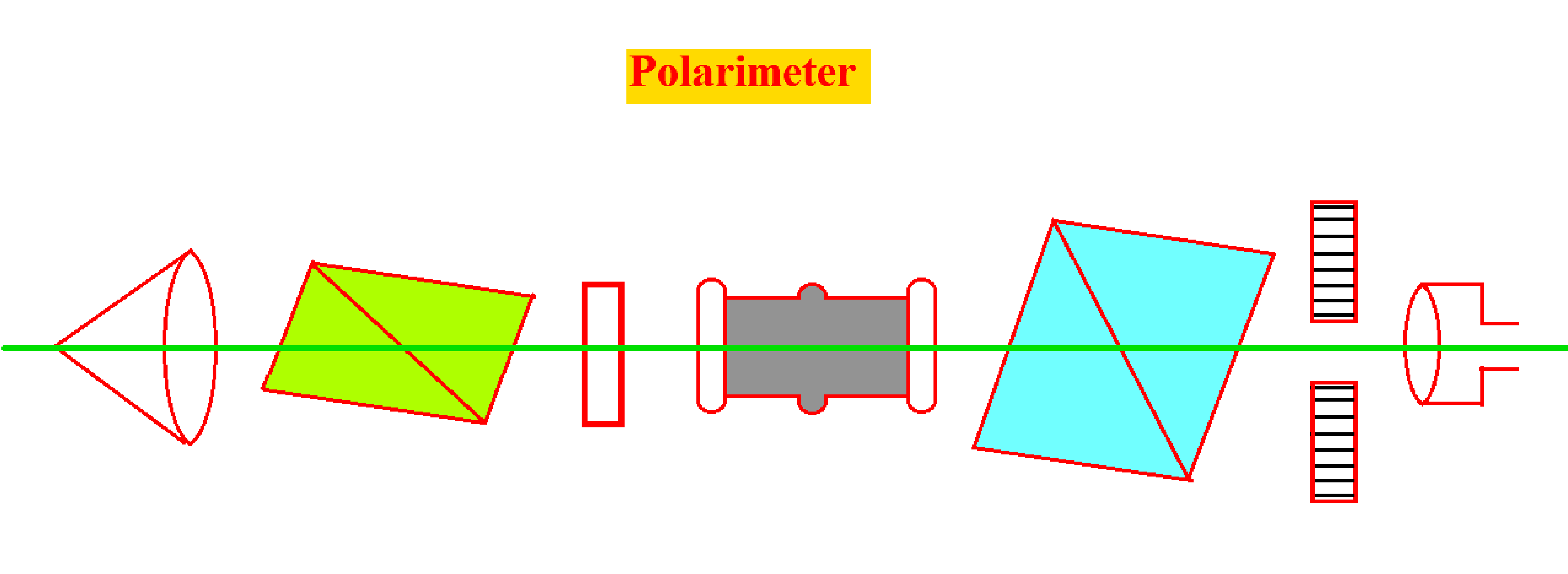
Laurentz Polarimeter
- Laurentz Polarimeter is the simplest form of Polarimeter and consists of two Nichol prisms known as Polarizer (P) and Analyzer (A).
- The Polarizer prism (P) is fixed, and Analyzer Prism (A) has the ability to rotate in its axis.
- The position of Analyzer Prism (A) can be measured by using a vernier caliper by moving over a circular path.
- The tube called the Pol tube is fixed co-axially in between the Polarizer prism and Analyzer Prism.
- The monochromatic light is allowed to pass through Lens (L) and through the polarizer through an empty tube.
- The Analyzer Nicol Prism (A) is gradually rotated in such a way that no light is transmitted. And the position of that prism is read on the scale and noted.
- Now both Polarizer prism and Analyzer Prism is used and the field is made dark.
- Now fill the tube with sugar solution, when the tube is completely filled it rotates in the plane of polarization of light passing through it.
- Darkness is again restored by slowly rotating the analyzer to the left or to right. Depending upon the type of solution used and the position on the scale is noted.
Industrial Polarimeter
- Monochromatic radiations from sodium lamps are made parallel by collimators and polarized by calcite prism.
- A small half-shade Nicol prism is placed next to the polarizer and arranged to intercept half of the beam.
- The Light radiation is made to pass through this Pol tube containing the sample of known length closed at both ends by glass plates and then through the analyzer to the eyepiece for visual observation.
- Without using a small prism the Polarimeter may function. The polarizer may initially be crossed without any sample in the beam and with the sample also.
- Addition of half prism avoids the possibility of difficulty.
- It is fixed with its polarizing axis and an angle of a few degrees from the polarizer.
pH Measurement
- pH measurement is a type of measuring technique used to find the nature of a substance whether it is an acidic or basic substance.
- Basically, it indicates the activeness of the substance to react, to know about the stability of a compound.
- There are various methods available to determine the pH of a substance by using portable instruments to know the actual pH value of the substance immediately.
- The term pH is widely used in the field of chemistry, Biochemical, and all organic and inorganic concepts.
- Element hydrogen consists of single highly active electrons. It tends to react with other elements to get a paired electron.
- Determination of the pH value of a material is done by two methods. 1. Electrometric method. 2. Colorimetric method.
- An accurate pH value of a substance can be determined by using the electrometric method but requires some special apparatus or equipment.
- But, the colorimetric method is simple and requires less expensive apparatus, which is sufficiently accurate for general work.
- This is subjected to interference by color, turbidity, high saline content, free chlorine, and various oxidants and reductants.
- This electrometric method shall be considered as the reference method to determine pH.
Electrometric Method
- The determination of pH can be made with any pH meter provided with a glass electrode, following instructions provided by the manufacturer. define the observed value to the nearest value of 0.1 unit.
- An electronic pH measurement system consists of
- Measuring Electrode – A measuring electrode is a glass electrode made of a thin glass membrane of special composition to develop potential proportional to the difference in Hydrogen ion concentration of liquid on either side of the membrane.
- The glass envelope has a pH-sensitive glass membrane at the bottom that contains a buffer solution of constant pH value.
- This measuring electrode is dipped in the measuring solution so that the potential developed at the platinum electrode is proportional to the pH of the measuring solution.
- The electrode potential can be calculated by completing the circuit with a reference electrode.
Reference Electrode
- The calomel electrode is considered a reference electrode in this measurement.
- This reference electrode consists of a glass envelope that contains a glass tube and calomel (mercury or mercurous chloride) solution along with platinum wire dipped in it.
- This tube is surrounded by a potassium chloride solution that slowly diffuses or leaks into process liquid through a liquid junction provided by asbestos fiber.
- A constant potential is developed by this reference electrode.
Potential Measuring System
- Here the measuring electrode and the reference electrode together form an electrolytic cell whose output is equal to the sum of the voltage developed by the two electrodes.
- This total voltage is applied to a null balance mill-volt potentiometer to calibrate the slide wire in terms of the pH of the measuring liquid
- Since the operation of the electrode depends upon the electrical resistivity of the glass, a change in temperature may cause an error in reading the pH value.
- To compensate for a change in temperature of the measuring solution a temperature compensation resistance is included in the circuit immersed in the solution.
- The resistance of this resistor varies with a change in the temperature of the measuring liquid.
Colorimetric Method
Reagents:
- This method requires a series of indicators and buffer solutions.
- The procedure for preparing an indicator is given below.
Procedure:
- Pour about 100ml of the sample into a glass tube.
- Use the universal indicator to calculate the approximate pH value
- Repeat the same by using an indicator solution of about 1/20th of the volume of the test liquid that corresponds to the approximate pH value.
- Compare the color that has been produced with a series of buffer solutions of known pH each containing the same proportion of the indicator.
- Note down the pH value such that the pH of the buffer solution must match the sample to the nearest 0.1 unit.
- For the determination of pH use some indicators to calculate its magnitude (value) based on color change.
Universal Indicator:
- To prepare a universal indicator dissolve 0.05g of methyl orange, 0.15g of methyl red, 0.3g of bromothymol blue, and 0.35g of phenolphthalein in one-liter alcohol(66 percent) which may be ethanol.
- In addition to 2 to 3 drops of the universal indicator with the sample, the change in the color will occur to determine the pH
- Compare the obtained color with the table shown below to know the exact pH value of the solution.
| pH Value | Color |
| Up to 3 | Red |
| 4 | Orange-red |
| 5 | Orange |
| 6 | Yellow |
| 7 | Yellowish-green |
| 8 | Greenish-blue |
| 9 | Blue |
| 10 | Violet |
| 11 | Reddish-violet |
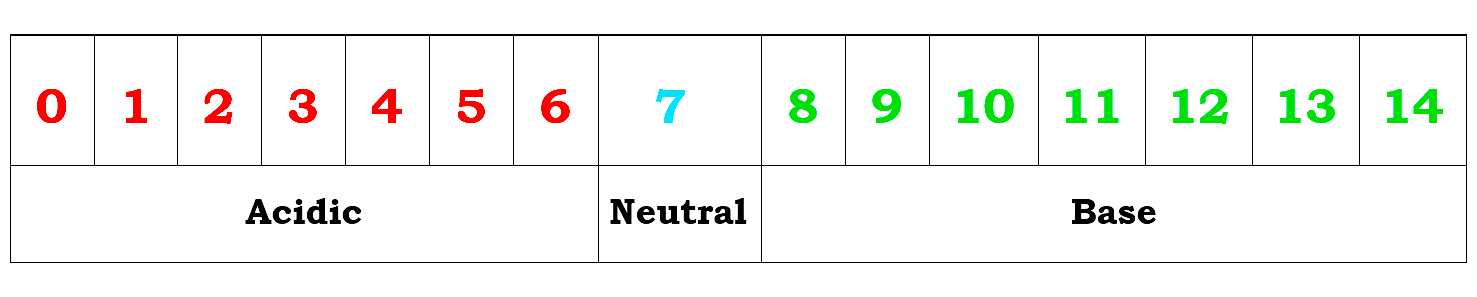
The pH of an aqueous solution is defined as the negative logarithm of its hydrogen ion concentration i.e.
PH = – log {H+}
pH Scale
Brix Measurement
- Brix measurement is a measure of the actual sucrose content present in cane juice, syrup, and in other fluids.
- Brix measurement is important and is required in many food processing industries such as the sugar industry, juice processing unit, soft drink plant, and other food processing areas that include sweeteners.
- Brix is referred to as the weight of sucrose content in a present in the solution.
- The Brix is measured on a degree scale ranging from 1-100.
- A solution, which is 40 °Brix, is 40 percent sucrose by weight.
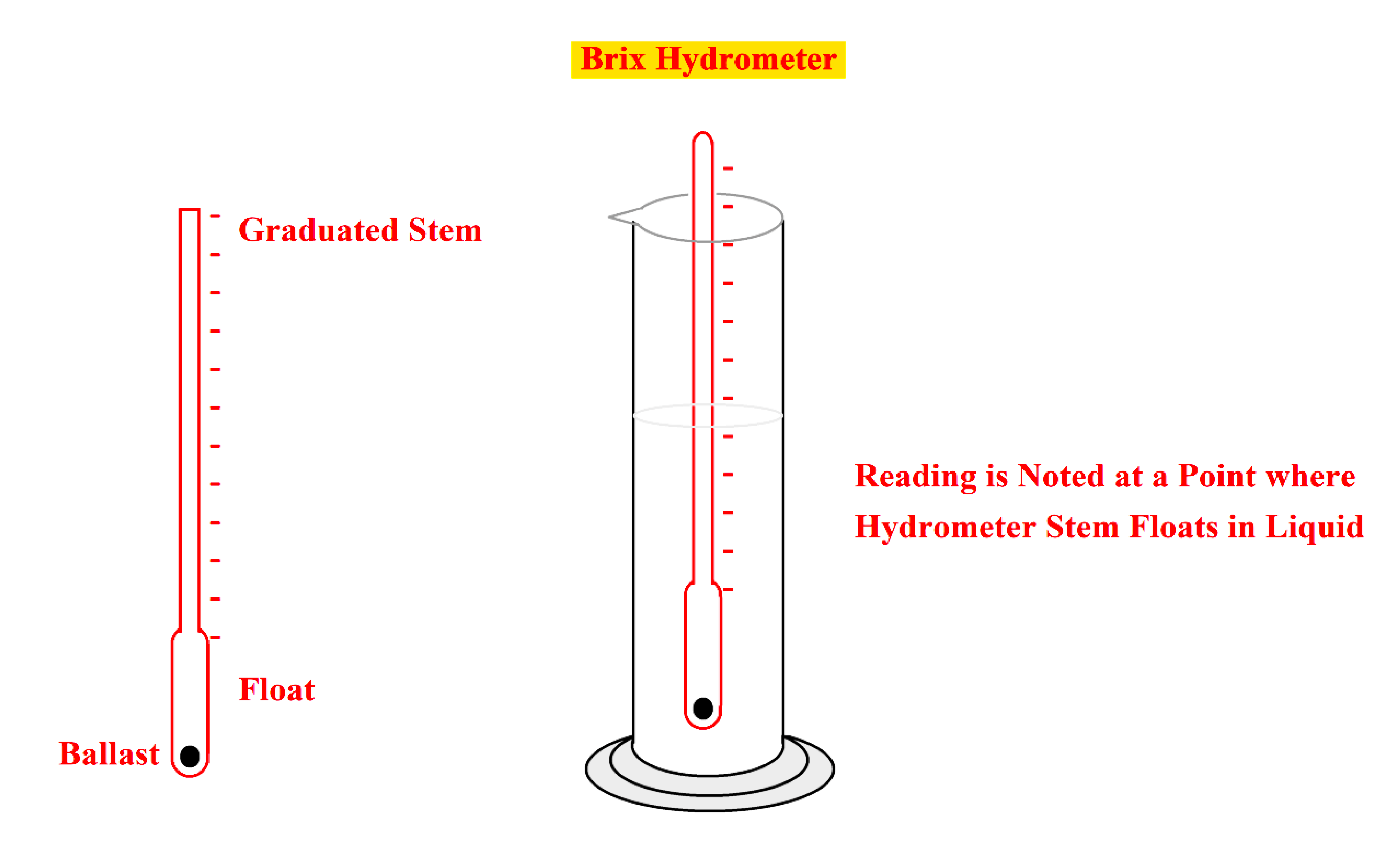
- The traditional method for the determination of the Brix is by constant weight hydrometer.
- The constant weight hydrometer functions on the law of the floating body displacing its own weight in a fluid.
- The hydrometer is made to float in a fluid and the density (specific gravity) of the fluid is determined by the fluid level on the scale of the stem.
- When a hydrometer is immersed in a liquid, The volume of the liquid displaced is given by
V= [(A*X) + W]
Let
V= volume of the stem to its base
A = cross-sectional area of the stem
W = weight of the hydrometer
R = Density of liquid
X= length of the stem immersed
- This Brix hydrometer determines the density and Brix of a sucrose solution.
- The Brix saccharometer is a hydrometer graduated to indicate the percentage of sucrose by weight at a given temperature for that hydrometer.
- Since the equation shown above involves the volume of liquid displaced.
- The measurement must is conducted considering the reference temperature. The reference temperature for specific gravities as they relate to Brix is 20 degrees Celsius.
- The impact of temperature on specific gravity and Brix can be seen in the following example.
- The amount of sucrose in the solution is 55 percent by weight.
- The change in temperature of the solution from 20° to 30°C causes a reduction in specific gravity due to the increase in the volume of the solution.
- The effect of temperature on the solution and specific gravity is eliminated by making measurements at or referencing 20° C.
55 lbs. Sucrose + 45 Lbs. H2O = 55 Brix solution
Coriolis Brix Principle of Operation
- The Coriolis meter works on the principle that the period of oscillation of the flow tube is related to the density of the liquid in the flow tube.
- conduct the same hydrometer measurement as the primary measurement
- The Coriolis meter determines the Brix of the solution as a function of the specific gravity of the solution.
- The process fluid is made to pass through the vibrating tube element.
- An electrical signal output from a sensing coil fixed on the flow tube defines the available frequency of oscillation.
- The period of oscillation is in proportion to the density of the liquid in the flow tube.
- The signal from the temperature sensor mounted on the flow tube is used to compensate for the changes in the tube’s modulus of elasticity due to temperature changes in the process liquid.
Benefits
- Coriolis density measurement yields highly accurate in-line Brix measurements.
- Coriolis meters offer a cost-effective measurement.
- This Coriolis meter is considered an extreme flow meter with high accuracy.
- The user benefits from two measurements in a single device since the device consists of a mass flow meter and a Brix meter.