Study about different Gas Analyzers Interview Questions and answers like NDIR analyzers, H2S Analyzer, Moisture Analyzer, HCDP Analyzer, CO2 Analyzer.
Gas Analyzers Questions
What is a Gas Analyzer?
Gas Analyzer is an instrument used to measure the concentration of a known gas in given mixture of gases from a process / stream.
Why are Gas Analyzer Used?
Gas Analyzer is used to Monitor Process, Enhance Safety, Increase Efficiency, Monitor Emission and Improve Quality.
Where are Gas Analyzer used?
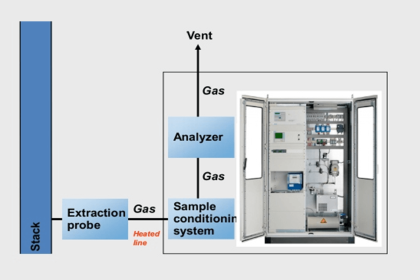
They are used in applications in all major industries such as Refineries & Petrochemicals, Chemical Plants, Critical process, Inerting Applications, pharmaceuticals, Fertilizers, Glass, semiconductors, Boilers, Heaters, Thermal crackers, Incinerators, continuous monitoring of Stack, Industrial gas producers and its users
What is the difference between Gas Detectors and Analyzers?
Detectors detect situations outside normal operating parameters and are set up to alarm.
Analyzers determine in real time, the quantity/concentration a said gas is in the stream / Process.
What is the different measurement techniques used in Gas Analyzers?
There are many different sensors used to analyze gases.
Types of popular sensors include Electrochemical, Paramagnetic, Thermal conductivity, Infrared, PID, FID etc.
Why are different kinds of sensors used?
The sensors are designed to detect / measure the unique physical or chemical properties of gases.
Hence to distinguish between different gases in a mixture of gases (a process or a stream) different sensor techniques are used.
Further Sensor choice also depends upon required accuracy, specifications, life expectancy and cost.
Does Gas Analyzer equipment need maintenance and calibration?
Yes. However in a properly Designed and commissioned system Maintenance requirement is minimal and may only consist of a visual inspection and verification of operative parameters and periodic replacements of consumables.
Calibration frequency depends on sensor type, criticality of application and accuracy requirement of the process.
What is calibration?
Calibration verifies that the Analyzer is operating properly and adjusts for any sensor drift or loss of sensitivity.
The process involves passing two known certified concentration of the target gas
- one for Low/Zero Point and other for High/Span Point
- usually from a calibration gas cylinder and allowing the Analyzer to adjust for drift in the reading.
What is cross-sensitivity?
Cross-sensitivity refers to the response of a sensor to a gas other than the target gas (also called an interference gas).
What is Nox?
A group of compounds mostly formed with Nitrogen and Oxygen as a byproduct of combustion are called NOX – Nitric Oxide (NO), Nitrogen Dioxide (NO2), Nitrous Oxide (N2O), N2O3, N2O4, N2O5, N3O4 and NO3.
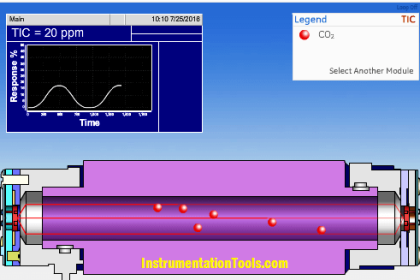
What does PPM mean?
Parts Per Million – 1 % volume = 10,000 ppm.
What is %LEL and %UEL
The primary risk associated with combustible gases and vapors is the possibility of explosions. Explosion, like fire, requires three elements: fuel, Oxygen, and an ignition source.
Each combustible gas or vapor will ignite only within a specific range of fuel/Oxygen mixtures. Too little or too much gas will not ignite. These conditions are defined as the Lower Explosive Limit (LEL) and the Upper Explosive Limit (UEL).
Any amount of gas between the two limits is explosive. It is important to note that each gas has its own LEL and UEL, as shown in the chart below. The gas concentrations are shown by percent of total volume, with the balance as normal air.
Between these two limits explosions can occur under some conditions, with the maximum explosive energy available at approximately the midpoint.
Note that these limits are sometimes referred to as LFL (Lower Flammable Limit) and UFL (Upper Flammable Limit).
These limits are empirically determined, and various authorities sometimes quote slightly different figures, based on slightly different experimental procedures.
What are the physical fundamentals of IR gas analysis?
The molecular vibrations of many chemical compounds can be excited by energies that lie in the infrared wavelength range. Therefore, these substances absorb infrared radiation.
The absorption spectrum depends mainly on the structure of the molecule and thus the degrees of freedom for the movement of the molecular components, their mass, their compositions, their spacing and their binding forces.
Therefore, each substance has a characteristic absorption spectrum. For example, in carbon dioxide molecules can be excited bending and stretching vibrations.
Additionally rotational movements around different molecular axes are possible, which superpose the vibrational spectrum and generate their fine structures.
How does a gas analyzer with an infrared spectrometer (IR spectrometer) work?
An IR spectrometer measures the absorption spectrums of gases. The comparison with the spectrums that are stored in a database allows a qualitative and quantitative reference for the substance.
How does non-dispersive infrared gas analysis (NDIR gas analysis) work?
For this form of gas analysis, the spectral sensitivity of a broadband thermal detector is limited by an optical bandpass filter. This is done for the range in which the absorption bands are found for the gas to be determined.
With the thermal detector, the transmission of the measured gas mixture is determined in a defined arrangement. If the gas being searched for is not present, most of the infrared radiation will reach the detector and the signal will be at its maximum. If the concentration of the gas increases, absorption will also increase according to Lambert-Beer’s law, and the signal will reduce accordingly.
What advantage does the non-dispersive infrared gas analysis (NDIR gas analysis) have?
Compared to infrared spectrometers, the NDIR gas analysis is significantly cheaper.
However, their use is only possible if the gases to be measured are known, and their number is low.
Which gases are not suitable for analysis with non-dispersive infrared gas analysis (NDIR gas analysis)?
Noble gases consist only of individual atoms. In order to vibrate, however, at least one bond is required. Noble gases can therefore not be detected with this method.
In diatomic elemental gases such as oxygen (O2) or nitrogen (N2), only a few vibrational modes can be excited by infrared radiation, so that even here the method fails.
What are the main components of an NDIR gas analyzer?
An NDIR gas analyzer consists of an electrically or mechanically modulated infrared source, a gas cell and usually a pyroelectric detector. An electronic device calculates the gas concentration based on signal voltage.
It offers a large number of standard IR narrow bandpass filters (NBP), which are optimally matched to the absorption properties of the gases to be measured.
The filters are mounted in the cap of the detector, which is welded to the detector base body. With multiple gases to be measured, the use of multi-channel detectors is recommended.
Are there advantages of having an absorption-free reference channel for non-dispersive infrared gas analysis (NDIR gas analysis)?
Yes, for NDIR gas analyzers, it is advisable to use a reference channel and normalize the signal of the gas duct via the quotient process on this reference.
The optical, mechanical and electronic drift of the overall system is reduced considerably, and the interval between calibrations can be significantly extended. The spectral position of the optical reference should be located as close as possible to the spectral lines of the gases to be measured.
A reference channel can be shared for multiple gases, if all absorption bands lie within a spectral window from 3 to 5 µm or from 8 to 12 µm. Without an optical reference channel, a reference can still be done by periodically introducing a reference gas into the channel, such as nitrogen.
How does an infrared flame sensor work?
The pyroelectric detector of the flame sensor detects the typical spectral radiance of burning organic materials such as wood, natural gas, oil or plastic.
In order to prevent a false alarm due to sunlight or other intense light sources, such as light from arc welding, two independent criteria of a flame are analyzed: First a typical flame is characterized by a flicker frequency of 1 to 5 Hz.
Secondly, a hydrocarbon flame contains the combustion gases carbon monoxide (CO) and carbon dioxide (CO2). Their emission bands lie in the infrared spectral range of 4.0 to 4.8 µm.
In order to obtain a high signal, one uses wide bandpass filters for the detector window, which include both the radiation emission of CO and of CO2. Optionally, a further channel can be used to recognize a further combustion by product, water.
OSHA measurers in PPM, what is that in percent?
- 10% is 100,000 PPM,
- 1% is 10,000 PPM,
- 0.5% is 5,000 PPM,
- 0.1% is 1000 PPM and
- 0.01% is 100 PPM.
Also Read: Calibration of Gas Detectors












Good. Thanks
Dear Sir,
With good approach to equipment I have enjoyed so far,Please I also need your full contribution to this instrument – HOT GAS GENERATOR mainly used in cement factories. The comprehensive knowledge of it.
Dear Sir,
With good approach to equipment I have enjoyed so far,Please I also need your full contribution to this instrument – HOT GAS GENERATOR mainly used in cement factories. The comprehensive knowledge of it.
Details and comprehensive operation of this HOT GAS generator can be send to my platform if its not available to all, I’ll be grateful. Thanks