Once the basic theory behind the operation of batteries is understood, we can apply these concepts to better understand the way batteries are utilized.
Series Cells
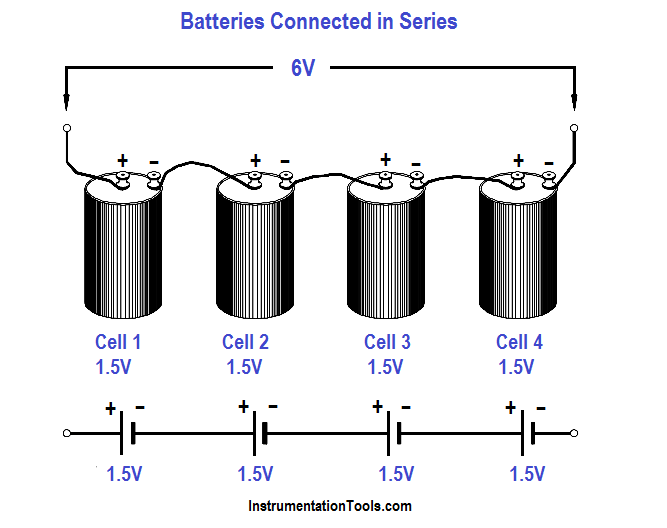
When several cells are connected in series (Figure 7), the total voltage output of the battery is equal to the sum of the individual cell voltages. In the example of the battery in Figure 7, the four 1.5V cells provide a total of 6 volts.
When we connect cells in series, the positive terminal of one cell is connected to the negative terminal of the next cell. The current flow through a battery connected in series is the same as for one cell.
Figure 7 : Cells Connected in Series
Parallel Cells
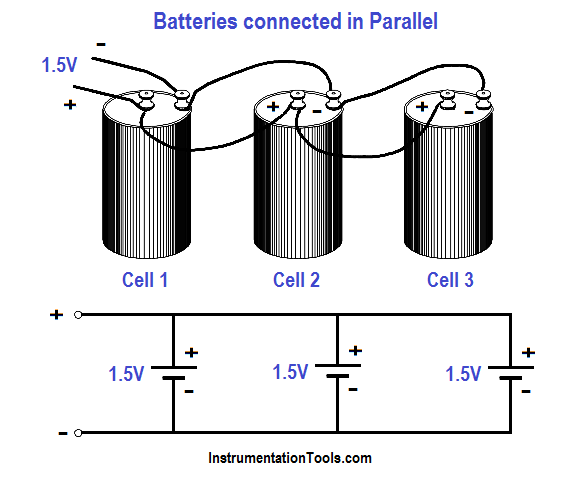
Cells connected in parallel (Figure 8), give the battery a greater current capacity. When cells are connected in parallel, all the positive terminals are connected together, and all the negative terminals are connected together. The total voltage output of a battery connected in parallel is the same as that of a single cell.
Cells connected in parallel have the same effect as increasing the size of the electrodes and electrolyte in a single cell. The advantage of connecting cells in parallel is that it will increase the current-carrying capability of the battery.
Figure 8 : Cells Connected in Parallel
Primary Cell
Cells that cannot be returned to good condition, or recharged after their voltage output has dropped to a value that is not usable, are called primary cells. Dry cells that are used in flashlights and transistor radios (e.g., AA cells, C cells) are examples of primary cells.
Secondary Cells
Cells that can be recharged to nearly their original condition are called secondary cells. The most common example of a secondary, or rechargeable cell, is the lead-acid automobile battery.
Capacity
The capacity of a storage battery determines how long the storage battery will operate at a certain discharge rate and is rated in ampere-hours. For example, a 120 ampere-hour battery must be recharged after 12 hours if the discharge rate is 10 amps.
Internal Resistance
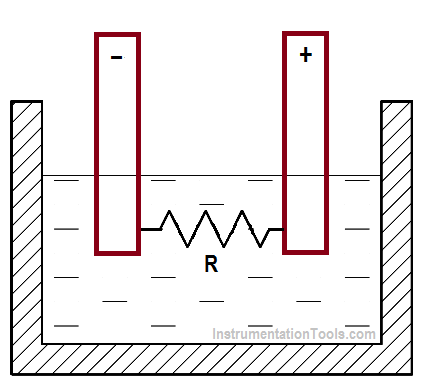
Internal resistance in a chemical cell is due mainly to the resistance of the electrolyte between electrodes (Figure 9).
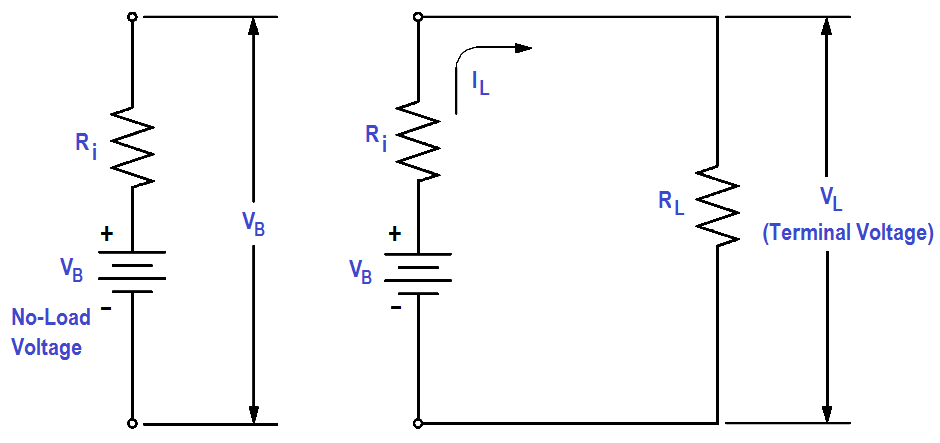
Any current in the battery must flow through the internal resistance. The internal resistance is in series with the voltage of the battery, causing an internal voltage drop (Figure 10).
With no current flow, the voltage drop is zero; thus, the full battery voltage is developed across the output terminals (VB). If a load is placed on the battery, load resistance (RL) is in series with internal resistance (Ri).
Figure 9 : Internal Resistance in a Chemical Cell
Figure 10 : Internal Voltage Drop
When current flows in the circuit (IL ), the internal voltage drop (ILRi) drops the terminal voltage of the battery as shown in below Equation. Thus, internal resistance reduces both the current and voltage available to the load.
VL = VB – ILRi
Shelf Life
The shelf life of a battery is the time which a battery may be stored and not lose more than 10 percent of its original capacity.
Charge and Discharge
The charge of a battery may refer to as one of two things:
- the relative state of capacity of the battery, or
- the actual act of applying current flow in the reverse direction to return the battery to a fully-charged state.
Discharge, simply stated, is the act of drawing current from a battery.
Battery Operations Summary
- The output voltage of a battery connected in series is equal to the sum of the cell voltages.
- A battery that is connected in parallel has the advantage of a greater current carrying capability.
- Secondary cells can be recharged; primary cells cannot be recharged.
- The unit for battery capacity is the ampere-hour.
- Internal resistance in a battery will decrease the battery voltage when a load is placed on the battery.
- Shelf life is a term that is used to measure the time that a battery may sit idle and not lose more than 10 percent of its charge.
- The charge of a battery may refer to one of two things: (1) the relative state of capacity of the battery, or (2) the actual act of applying current flow in the reverse direction to restore the battery to a fully-charged condition.
- Discharge refers to the act of drawing current from a battery.