Volume CH4: The percentage by volume of combustible methane gas in an area. At 5% volume the mixture of methane is at 100% LEL and will explode if combined with oxygen and a spark.
% Volume: Volume of a gas in air (i.e., 20.9% volume of oxygen is the standard percentage of oxygen by volume in air).
Absorption: The gas penetrates the solid surface by chemical reaction.
Adsorption: The collection of gas or liquid molecules on the outside or surface of another material.
Bump Gas: This is a term used in the industry as of just recently to check if the sensors respond to a gas. This gas IS NOT certified and most likely has an unknown concentration.
Calibration: This is a term used in the industry when an instrument is calibrated with a “certified” calibration gas.
Catalytic Combustion: A method used in LEL sensors to detect combustible gases. The sensor is made up of a Wheatstone bridge with two (2) matched pair sensing elements, one active and one passive. These elements are heated to a temperature of 1,000°F and balanced to zero without gas. When gas comes into contact with the elements, the active element heats to a higher temperature, changing the resistance in the circuit, and the value is displayed on the meter.
Clean Air: An atmosphere which contains < 1 ppm of total hydrocarbons in an atmosphere with 20.9% volume oxygen without any toxic gases. Nitrogen makes up the balance of air.
Combustible Gas: A gas or material that will burn or ignite when the proper mixture of air and heat combine. Each combustible gas (hydrocarbon) has a Lower Explosion Limit and will have a different flash point or volatility. Combustible gases are detected with an LEL sensor (catalytic combustion).
Difference Between % LEL and % Volume: The difference between % LEL and % volume is that the % LEL is a portion of the volume range. (i.e. 100% LEL for methane is equivalent to 5% volume).
Electrochemical Cell: A sensor that detects “toxic gases.” The electrochemical sensor will produce an output when it comes into contact with the toxic gas that the sensor is specified for.
Fuel Cell: The oxygen sensor is a “fuel cell.” It generates a constant electrical output just like a battery. The oxygen sensor (selfdepleting) can be consumed in or out of the instrument.
IDLH: Immediately Dangerous to Life and Health is the maximum concentration of gas that a person can not withstand for 30 minutes without symptoms or irreversible health problems. This is also used to determine the proper selection of a respirator.
IR: Stands for infrared. These sensors are one of the most specific sensors in the industry; however, there are still some interfering gases that can cause a false reading. This is the preferred sensor for detecting CO2 in the atmosphere from the ppm to % volume range along with certain hydrocarbons in inert (oxygen-free) atmospheres.
LEL: The Lower Explosion Limit is the lowest concentration of a gas or vapor (% volume in air) that bums or explodes if an ignition source is present at ambient (room) temperatures.
M.O.S.: A Metal Oxide Semiconductor is used and will pick up most organic vapors in the atmosphere. This sensor can be a lifesaver when used properly in a confined space instrument as a general detector for unknown gases. Also known as a “broad range sensor,” it will detect more gases than the currently popular “P.I.D.” instrument.
Organic Vapors: Compounds composed of carbon, hydrogen, and other elements with a chain or ring structure.
Oxygen Deficiency: An atmosphere that contains less that 19.5% volume oxygen. You can suffocate to death in air with low concentrations of oxygen.
Oxygen Enrichment: An atmosphere that contains more than 23.5% volume oxygen. The hazard of enriched oxygen is fire; high levels of oxygen make ignition much easier.
Oxygen: Oxygen in the air, measured in % volume. 20.9% volume is normal.
P.I.D.: A Photo Ionization Detector is a gas detector typically used to detect low concentrations of gases from 0.1 to 2,000 ppm. They are used as leak detectors in plant surveys to identify potential problem areas and to determine the need for PPE. These instruments are nonspecific if more than one compound is in the air and have more limitations than the M.O.S. sensor.
PEL: OSHA established Permissible Exposure Limit. This may be expressed as the TWA limit or as a ceiling exposure limit that LEGALLY must never be exceeded, even if the TWA exposure is not violated. OSHA PELs have the force of law!
ppm: Parts per million (i.e., 10 ppm H2S is 10 parts of a million).
Reactive Gas: This is a gas that is usually unstable, i.e. hydrogen sulfide or chlorine.
STEL: The Short Term Exposure Limit is a 15 minute average that should never be exceeded at any time during a workday. There is a maximum of four such periods per day, with at least 1 hour between exposure periods, provided that the daily TLV or TWA is not exceeded.
TLV: The Threshold Limit Value is the same as the TWA.
TOXIC GAS: Any gas defined by the government as harmful to breathe; measured in ppm (parts per million). Many of these gases are also combustible at some level.
Toxic Gas: Any chemical or material that has evidence of causing an acute or chronic health hazard and is listed in the NIOSH registry, provided that the substance causes harm at any dosage level.
TWA (TLV): Time Weighted Average or Threshold Limit Value of a gas. The government has set levels of toxic gas that workers can be exposed to over a period of time (i.e., the average exposure under which a worker can perform safely for a normal 8 hour workday, 40 hours per week) without any harmful effects.
UEL: The Upper Explosion Limit is the highest concentration of a material in air that produces an explosion or ignites when it contacts an ignition source (high heat, electric arc, spark, or flame). A higher concentration of material in a smaller concentration of air may be too rich to ignite.
Zeroing: Zero means there should be NO GAS present in the area. When you “zero” the instrument, the instrument reading displays “Zero.” You must make sure that the atmosphere is free from gas; if not, use a zero gas that provides air with total hydrocarbons less than 1 ppm.








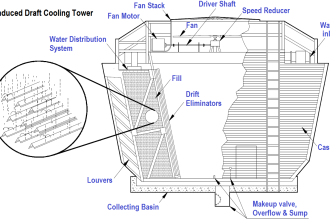
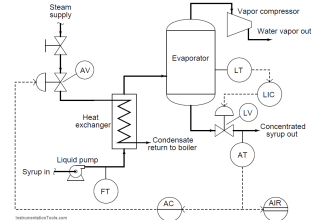



Dear Sirs
Hope you are all doing well.
I am very much thankful to you all for sending these information of gas detection instruments.
Please send me as much information as you all can send as it is increasing my knowledge.
Regards
Syed Abdul Hafeez Basha
all given tech. information are valuable , appreciate very much yr efforts.