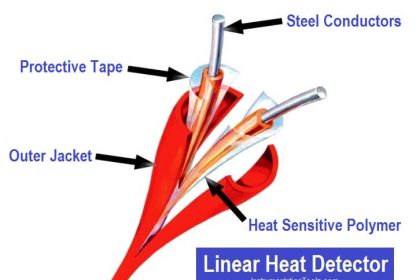
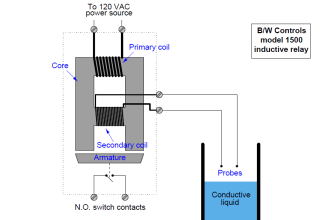
Schematic diagrams of Electrochemical-type gas sensor and Chemical reactions
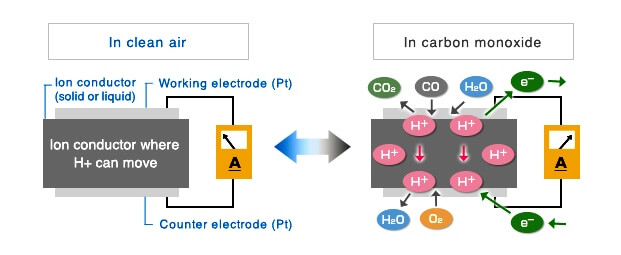
Image Credits : figaro
Electrochemical-type gas sensor are amperometric fuel cells with two electrodes. The basic components of two electrode gas sensors are a working (sensing) electrode, a counter electrode, and an ion conductor in between them. When toxic gas such as carbon monoxide (CO) comes in contact with the working electrode, oxidation of CO gas will occur on the working electrode through chemical reaction with water molecules in the air (see Equation 1).
CO + H2O → CO2+ 2H+ + 2e– …(1)
Connecting the working electrode and the counter electrode through a short circuit will allow protons (H+) generated on the working electrode to flow toward the counter electrode through the ion conductor. In addition, generated electrons move to the counter electrode through the external wiring. A reaction with oxygen in the air will occur on the counter electrode (see Equation 2).
(1/2)O2 + 2H+ + 2e– → H2O …(2)
The overall reaction is shown in Equation 3. Figaro Electrochemical-type gas sensor operate like a battery with gas being the active material for this overall battery reaction.
CO + (1/2)O2 → CO2 …(3)
By measuring the current between the working electrode and the counter electrode, we can measure the equivalent measured gas readings. So this electrochemical cell can be utilized as a gas sensor.
Also Read : F & G Loop Check Procedures
Theoretical equation for CO detection
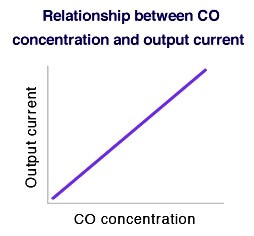
In order to measure the sensor’s output current, it must be connected to an external circuit. By controlling gas flowing toward the working electrode with diffusion film, output current flowing across the external circuit will be proportional to gas concentration (see Equation 4 and the chart at the right). The linear relationship of gas concentration to sensor output makes this technology ideal for gas sensing applications.
I = F × (A/σ) × D × C × n …(4)
where:
I: Sensor output
F: Faraday constant
A: Surface area of diffusion film
σ:Thickness of diffusion film
D: Gas diffusion coefficient
C: Gas concentration
n: Number of reaction electrons
Credits : figaro








THANK YOU for this article
if i have a gas sensor with 3 wires black white and red ,
how do i check my sensor if it is really damaged or not ?
some say you measure the resistance between each two wires and you find the abstract equals ZERO