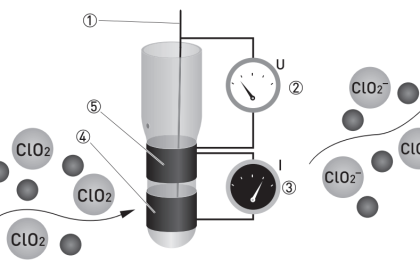
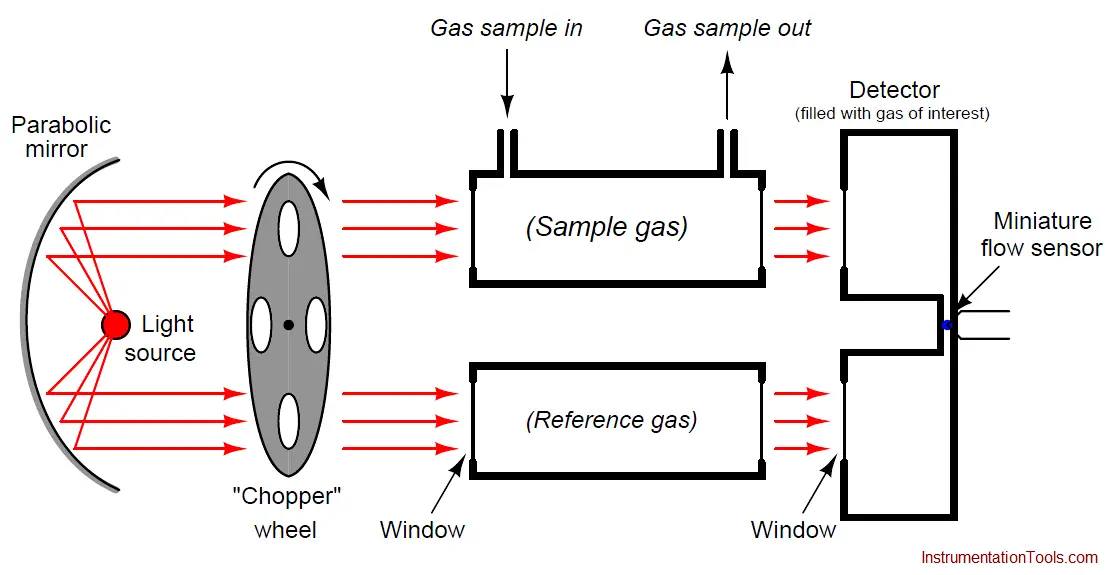
A very clever way to achieve selectivity with a non-dispersive optical analyzer is to replace the thermopiles with a detector more sensitive to the wavelengths absorbed by the gas of interest than to the wavelengths absorbed by any other (“interfering”) gas species. Dr. Luft invented just such a detector when developing the NDIR gas analyzer for I.G. Farben in the late 1930’s. His design used two gas chambers and a thin diaphragm to measure the difference in light intensity exiting the sample and reference cells:
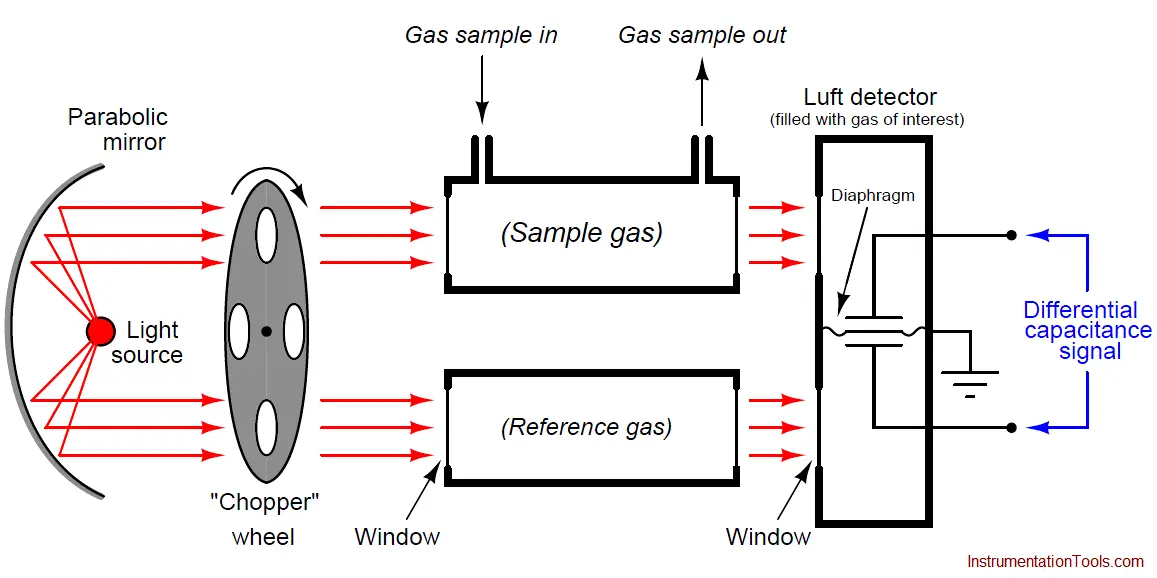
As light enters the dual chambers of the detector, the light absorbed by the fill gas causes those gas molecules to increase temperature. This expands the gas, pressing against the diaphragm. If the light intensities are equal, the pressures will be equal and no diaphragm motion will result. If the light intensities are unequal (due to the sample cell absorbing some of the wavelengths), the gas pressure developed inside that half of the Luft detector will be less, causing the thin diaphragm to bow in that direction. A set of fixed metal plates senses the diaphragm’s position using the differential capacitance technique (just like many modern differential pressure sensors). With the “chopper” wheel working to pulsate light through the sample and reference gas cells, the diaphragm motion will likewise pulsate, and the resulting “AC” pulse signal may be filtered and amplified to represent absorbing gas concentration.
What makes the Luft detector selective is that it is filled with a 100% concentration of the gas we are interested in measuring. This means only those wavelengths of light absorbed by the gas of interest will develop heat (and pressure) inside the detector chambers. Different wavelengths of light absorbed by other (“interfering”) gases in the sample will not be absorbed to the same degree (or at all) by the gas inside the Luft detector, and therefore the pressure pulses inside the Luft detector will be primarily a function of our interest-gas concentration and not of the interfering-gas concentration(s).
The selectivity gained by a gas-filled Luft detector is not obvious to see at first, and deserves some explanation. We may explore this selective behavior in more detail by performing a set of “thought experiments” whereby we imagine the effects of different gas species on an NDIR analyzer equipped with a Luft detector.
Suppose we have an application where we intend to measure carbon dioxide concentration in a gas mixture also containing ethane. In a simple dual-beam NDIR detector using thermopile detectors, both carbon dioxide and ethane present in the sample chamber will generate a detector response, since both gas species absorb infrared light, and the thermopile detectors respond to any change in the amount of infrared light received at the detector. Thus, such a simple analyzer could not tell the difference between a change in carbon dioxide concentration versus a change in ethane concentration. This makes ethane an “interferent” given our goal of only measuring carbon dioxide concentration.
While both carbon dioxide and ethane gases absorb infrared light, they do so at different specific wavelengths. The following spectral plots show the unique infrared absorption bands for carbon dioxide and ethane, respectively. As you can see, the wavelengths of infrared light absorbed by each species of gas are unique, and do not overlap:
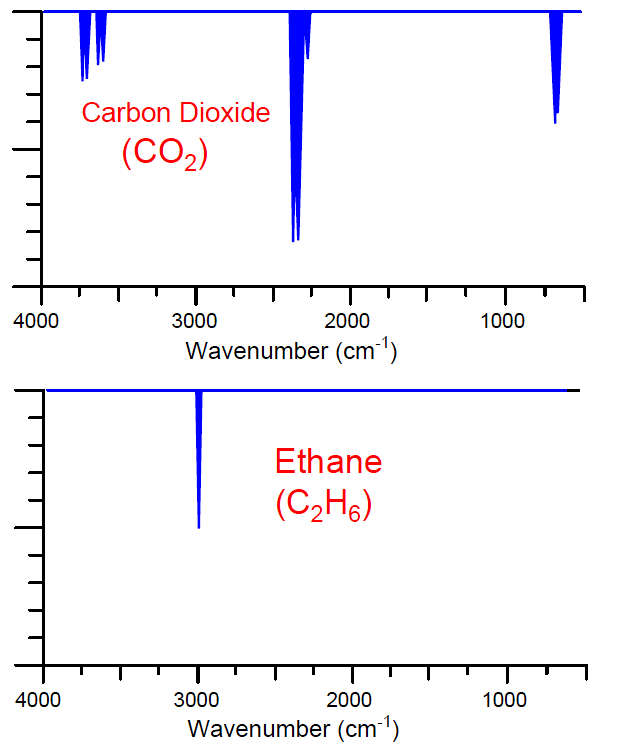
Let us now imagine replacing the thermopile detectors with a Luft detector, its dual chambers filled with a 100% concentration of carbon dioxide gas. If neither carbon dioxide nor ethane are present in the sample chamber, light will pass from the source undiminished to the Luft detector, causing equal heating of the CO2 gas in both chambers and therefore zero response. This is precisely what we would expect from any dual-beam NDIR instrument, Luft detector or not.
For our next “thought experiment,” imagine carbon dioxide gas entering the sample chamber. Those carbon dioxide molecules entering the sample chamber will absorb some of the infrared light emitted by the source. Since the carbon dioxide gas molecules inside the Luft detector can only be heated by those same wavelengths of light absorbed by the molecules in the sample chamber, the sample-side of the Luft detector will now experience less heating than before (while the reference-side experiences the same degree of heating), causing a difference in pressure inside the Luft detector and therefore generating a response. Once again, this is precisely what we would expect from any dual-beam NDIR instrument, Luft detector or not.
However, if we now imagine only ethane molecules entering the sample chamber, the Luft detector’s response will be different from that of the thermopile detector. Surely, the ethane molecules will absorb some of the infrared light entering that chamber, but these “missing” wavelengths will be of no effect at the Luft detector because they aren’t absorbed by the carbon dioxide gas inside the Luft detector chambers anyway, and therefore would not affect the temperature of the detector’s carbon dioxide gas whether missing or present. In other words, the gas-filled Luft detector “doesn’t care” about any wavelengths of light absorbed by gases in the sample chamber so long as the absorption pattern of the sample gas does not coincide at any point with the absorption pattern of the gas filling the Luft detector. This means the ethane’s attenuation of infrared light wavelengths will be ignored by the carbon-dioxide-filled Luft detector, while carbon dioxide’s attenuation of infrared light will be sensed by the Luft detector. We may now say that the instrument is “sensitized” to carbon dioxide gas, and that the Luft detector is “selective” to one species of gas over and above all other species.
If a mixture of carbon dioxide and ethane gases enters the sample chamber, each type of gas molecule will absorb its unique pattern of light wavelengths, but only the attenuation of those wavelengths matching the absorption pattern of the Luft detector’s fill gas will register in the detector. Thus, the Luft detector is able to selectively measure the concentration of one gas inside the sample chamber to the exclusion of all other gases having different optical absorption patterns.
A modern variation on the Luft detector design replaces Luft’s original microphone-style thin diaphragm with a narrow channel and a highly sensitive thermal flow sensor connecting the two gas-filled chambers. Any difference in expansion between the gases of the two chambers when heated by light causes gas to move past the flow sensor, thus generating a signal:
As the chopper wheel pulses incident light to either chamber of the detector, gas will flow back and forth through the narrow passageway connecting the two chambers, causing an alternating flow response from the flow sensor.
The advantage of a diaphragm-less detector is that it is just as insensitive to mechanical vibration as a thermopile (having no moving parts), but retains the spectral selectivity of the traditional Luftstyle detector (being filled with the gas of interest).
While Luft-style detectors greatly enhance the selectivity of non-dispersive spectrographic gas analyzers, there is still room for improvement. Perfect selectivity of measurement is assured by a Luft detector only when the light absorption spectra of the interference gas(es) do not overlap at all with the absorption spectrum of the gas of interest. If there is some overlap, interference will result.
To address this concern, we will explore one more design feature of modern non-dispersive analyzers: filter cells.