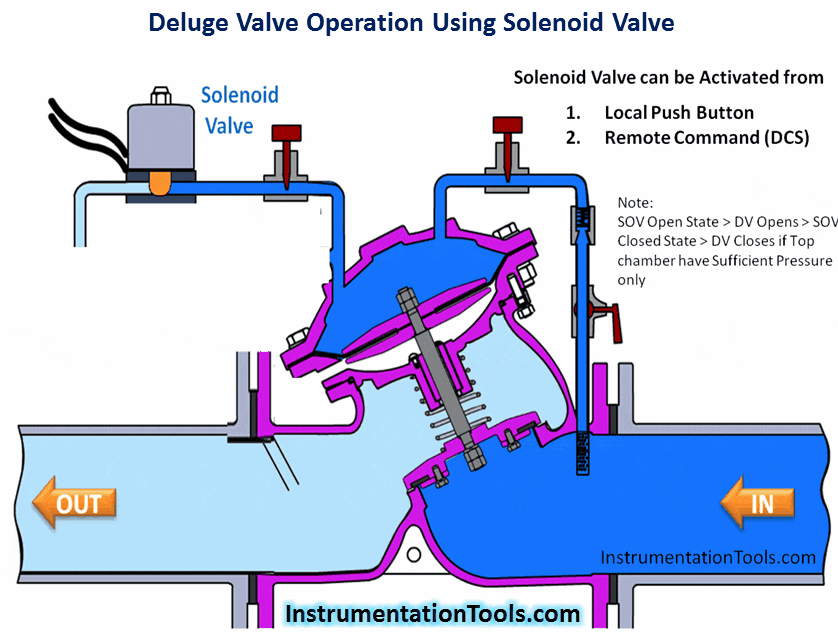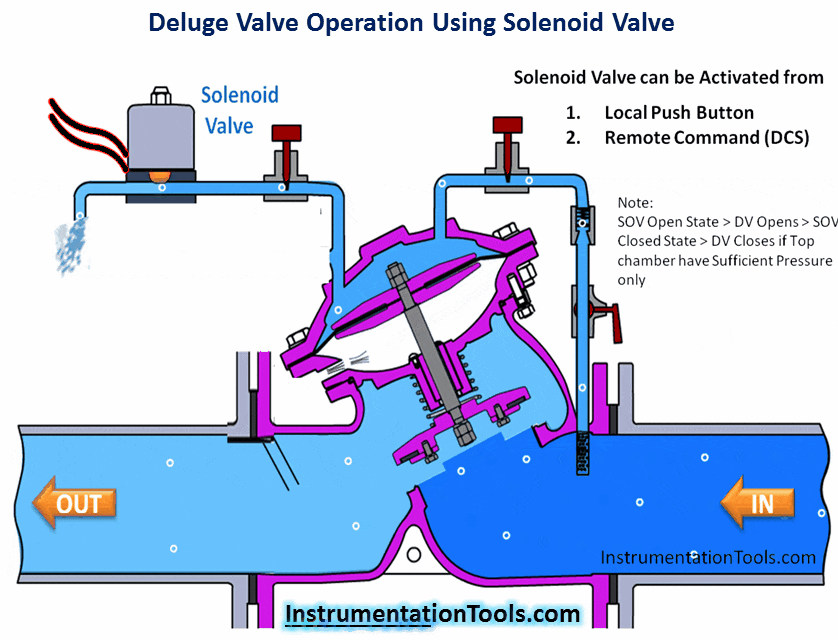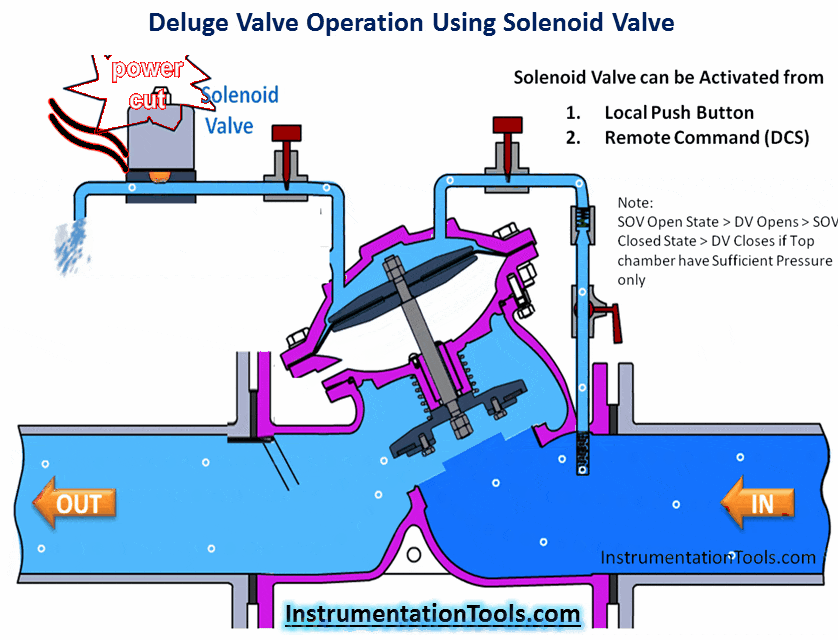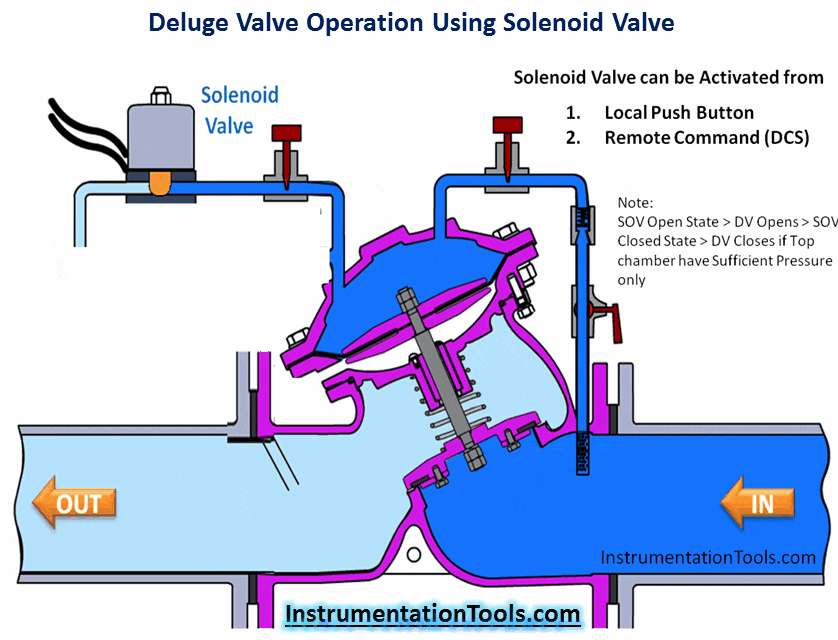
Hydrogen Sulfide (H2S) is a highly toxic gas, with a pungent “rotten eggs” odor at low concentrations but no visible color. At higher concentrations, the gas acts as a nerve agent to de-sensitize human smell, so that it seems odorless. Its paralytic effect on smell extends to more important bodily functions such as breathing, causing rapid loss of consciousness and asphyxiation.
A photograph of a safety chart (taken at a wastewater treatment facility) shows just how toxic hydrogen sulfide gas is:
Note how concentrations in the parts per million range are hazardous, and how little H2S concentration is required to paralyze one’s sense of smell. Hydrogen sulfide also happens to be flammable, its LEL value in air being 4.3%. However, the toxic properties of the gas are generally the more pressing concern when released into the atmosphere. Another hazardous property of hydrogen sulfide is its density: 1.18 times that of air. This means H2S gas will tend to collect in low areas such as pits, electrical vaults, and empty underground storage vessels.
The principal source of hydrogen sulfide gas is anaerobic (oxygen-less) decomposition of organic matter. Sewage treatment facilities, pulp mills, and oil refineries generate H2S gas in significant quantity, and so employees at such facilities must be continually aware of the associated hazards.
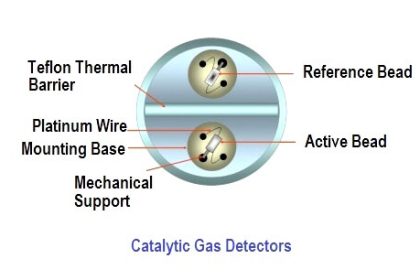
One of the most popular analytical sensing technologies for H2S gas appropriate for portable monitoring includes an electro-chemical reaction cell similar in principle to the micro fuel cells used to detect oxygen concentrations. Hydrogen sulfide gas entering such a cell engages in a specific chemical reaction, creating a small electrical current proportional to the gas concentration. Like oxygen-sensing fuel cells, these chemical cells also have limited lives and must be routinely replaced.
Overview of Hydrogen Sulfide (H2S)
Hydrogen sulfide is a colorless, flammable, extremely hazardous gas with a “rotten egg” smell. It occurs naturally in crude petroleum and natural gas, and can be produced by the breakdown of organic matter and human/ animal wastes (e.g., sewage). It is heavier than air and can collect in low-lying and enclosed, poorly ventilated areas such as basements, manholes, sewer lines and underground telephone/electrical vaults.
- Can be smelled at low levels, but with continuous lowlevel exposure or at higher concentrations you lose your ability to smell the gas even though it is still present.
- At high concentrations – your ability to smell the gas can be lost instantly.
- DO NOT depend on your sense of smell for indicating the continuing presence of this gas or for warning of hazardous concentrations.
Health effects vary with how long, and at what level, you are exposed. Asthmatics may be at greater risk.
- Low concentrations – irritation of eyes, nose, throat, or respiratory system; effects can be delayed.
- Moderate concentrations – more severe eye and respiratory effects, headache, dizziness, nausea, coughing, vomiting and difficulty breathing.
- High concentrations – shock, convulsions, unable to breathe, coma, death; effects can be extremely rapid (within a few breaths).
- The air needs to be tested for the presence and concentration of hydrogen sulfide by a qualified person using test equipment. This individual also determines if fire/explosion precautions are necessary.
- If gas is present, the space should be ventilated.
- If the gas cannot be removed, use appropriate respiratory protection and any other necessary personal protective equipment (PPE), rescue and communication equipment. Atmospheres containing high concentrations (greater than 100 ppm) are considered immediately dangerous to life and health (IDLH) and a selfcontained breathing apparatus (SCBA) is required.
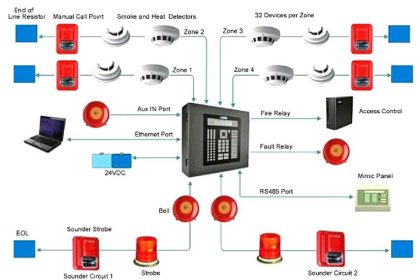
Also Read : Fire Detection and Alarm System