Steam drum water level measurement is actually a form of interface level measurement, because high- pressure steam is significantly denser than air under ambient conditions:
Steam Drum Water Level Measurement
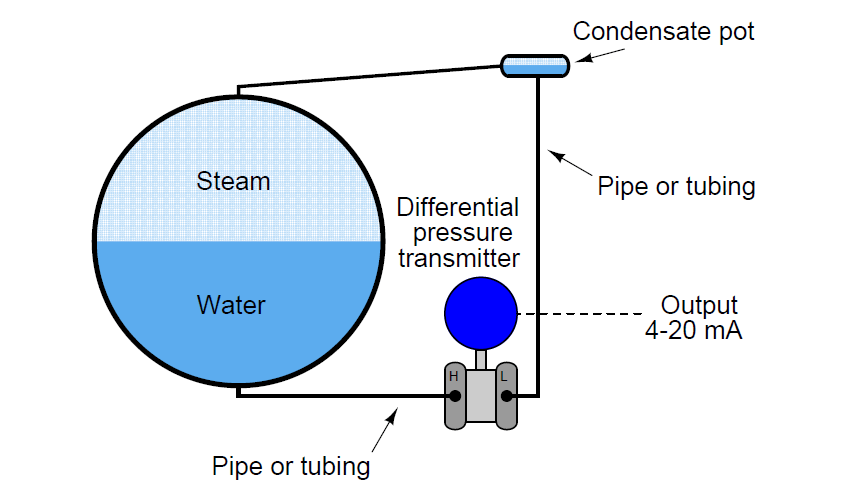
Making this situation even more complex is the fact that the densities of both the water and the steam change as boiler pressure and temperature change.
Identify what happens to water density and steam density as both pressure and temperature increase, and explain why.
Answer:
As boiler temperature increases, the water density decreases. For saturated steam conditions (i.e. water and steam in direct contact with each other in the same vessel), pressure and temperature are directly related.
So, as temperature in the steam drum increases, pressure must also. This increase in pressure causes the steam to become denser as the steam molecules become packed closer together.
Just to give you an example of how significant these density changes are at high pressure (e.g. power generation boilers), consider the following values:
Boiling (saturated) water density at 2,425 PSIG = 35.49 lb/ft3
Saturated steam density at 2,425 PSIG = 7.33 lb/ft3
Share your answers & explanation with us through the below comments section.
Read Next:
- Types of Tank Gauging
- Level Transmitter Errors
- Sump Tank Level Transmitter
- Magnetostrictive Level Sensor
- Cat and Mouse Level Gauge
Credits: Tony R. Kuphaldt