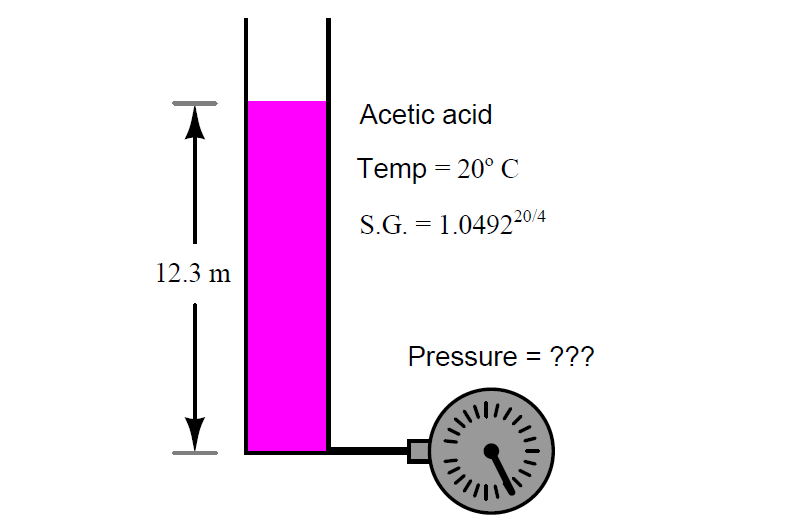
Acetic acid has a specific gravity of 1.049220/4.
Explain what the superscript “20/4” means, and calculate how much hydrostatic pressure a vertical column of acetic acid 12.3 meters high is supposed to produce, in PSI at 20o C.
Hydrostatic Pressure of a vertical column
P = ____________ PSI
Suppose the pressure gauge connected to the bottom of this vessel registers 14.2 PSI instead of the value you calculated.
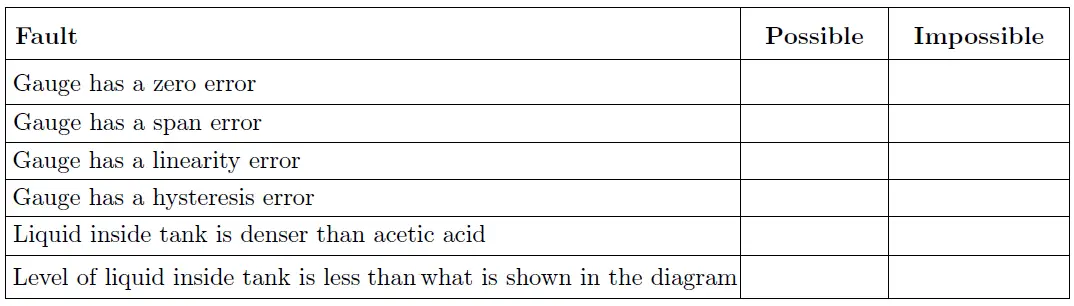
Identify the likelihood of each specified fault shown in the table, considering each fault one at a time (i.e. no coincidental faults) to determine whether or not each fault could independently account for the discrepancy in pressure measurement.
Answer:
The 20/4 superscript means is that acetic acid is this much denser at 20o C than water is at 4o C.
Questions For You:
1. Explain how we could determine what kind of calibration error this gauge had, if indeed its calibration was off at all.
2. Identify some alternative level-measurement technologies which could be used to sense liquid level in this vessel, which aren’t affected by changes in liquid density.
Share your answers with us through the below comments section.
Read Next:
- Motion-Balance Instrument
- PLC Pneumatic Circuit
- PSV Lift Pressure
- Air Compressor Problem
- DP Transmitter Pressure
Credits: Tony R. Kuphaldt