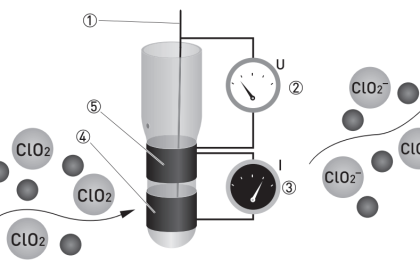
Conductivity is used to measure the ability of an aqueous solution to carry an electrical current. Specific conductance is the conductivity value corrected to 25 DC.
Most instruments are calibrated against a single standard which is near the specific conductance of the environmental samples. The standard can be either below or above the specific conductance of the environmental samples. A second standard is used to check the linearity of the instrument in the range of measurements.
When performing specific conductance measurement on groundwater or surface water and the measurement is outside the initial calibration range defined by the two standards, the instrument will need to be re-calibrated using the appropriate standards .
Specific Conductance Calibration Procedure:
1. Allow the calibration standards to equilibrate to the ambient temperature.
2. Fill calibration containers with the standards so each standard will cover the probe and temperature sensor. Remove probe from its storage container, rinse the probe with deionized water or a small amount of the standard (discard the rinsate), and’place the probe into the standard.
3. Select measurement mode. Wait ‘until the probe temperature has stabilized.
4. Select calibration mode, then specific conductance. Enter the specific conductance standard value. Make sure that the units on the standard are the same as the units used by the instrument. Ifnot, convert the units on the standard to the units used by the instrument.
5. Select measurement mode. The reading should remain within manufacturer’s specifications. If it does not, re-calibrate, If readings continue to change after re- calibration, consult manufacturer or replace calibration solution.
6. Remove probe from the standard, rinse the probe with deionized water or a small amount of the second standard (discard the rinsate), and place the probe into the second standard. The second standard will serve to verify the linearity of the instrument. Read the specific conductance value from the instrument and compare the value to the specific conductance on the standard. The two values should agree within the specifications of the instrument. If they do not agree, re-calibrate. If readings do not compare, then the second standard may be outside the linear range of the instrument. Use a standard that is closer to the first standard and repeat the verification. If values still do not compare, try cleaning the probe or consult the manufacturer.
7. After the calibration has been completed, rinse the probe with deionized water and store the probe according to manufacturer’s instructions.
8. Record the calibration information on calibration log sheet.
Note: for projects where specific conductance is not a critical measurement it may be possible to calibrate with one standard in the range of the expected measurement.