There are two types of pressurizers: static and dynamic. A static pressurizer is a partially filled tank with a required amount of gas pressure trapped in the void area. A dynamic pressurizer is a tank in which its saturated environment is controlled through use of heaters (to control temperature) and sprays (to control pressure).
This article focuses on the dynamic pressurizer. A dynamic pressurizer utilizes a controlled pressure containment to keep high temperature fluids from boiling, even when the system undergoes abnormal fluctuations.
Before discussing the purpose, construction, and operation of a pressurizer, some preliminary information about fluids will prove helpful.
The evaporation process is one in which a liquid is converted into a vapor at temperatures below the boiling point. All the molecules in the liquid are continuously in motion. The molecules that move most quickly possess the greatest amount of energy. This energy occasionally escapes from the surface of the liquid and moves into the atmosphere. When molecules move into the atmosphere, the molecules are in the gaseous, or vapor, state.
Liquids at a high temperature have more molecules escaping to the vapor state, because the molecules can escape only at higher speeds. If the liquid is in a closed container, the space above the liquid becomes saturated with vapor molecules, although some of the molecules return to the liquid state as they slow down. The return of a vapor to a liquid state is called condensation. When the amount of molecules that condense is equal to the amount of molecules that evaporate, there is a dynamic equilibrium between the liquid and the vapor.
Pressure exerted on the surface of a liquid by a vapor is called vapor pressure. Vapor pressure increases with the temperature of the liquid until it reaches saturation pressure, at which time the liquid boils. When a liquid evaporates, it loses its most energetic molecules, and the average energy per molecule in the system is lowered. This causes a reduction in the temperature of the liquid.
Boiling is the activity observed in a liquid when it changes from the liquid phase to the vapor phase through the addition of heat. The term saturated liquid is used for a liquid that exists at its boiling point. Water at 212 oF and standard atmospheric pressure is an example of a saturated liquid.
Saturated steam is steam at the same temperature and pressure as the water from which it was formed. It is water, in the form of a saturated liquid, to which the latent heat of vaporization has been added. When heat is added to a saturated steam that is not in contact with liquid, its temperature is increased and the steam is superheated. The temperature of superheated steam, expressed as degrees above saturation, is called degrees of superheat.
General Description
The pressurizer provides a point in the reactor system where liquid and vapor can be maintained in equilibrium under saturated conditions, for control purposes. Although designs differ from facility to facility, a typical pressurizer is designed for a maximum of about 2500 psi and 680oF.
Dynamic Pressurizers
A dynamic pressurizer serves to:
- maintain a system’s pressure above its saturation point,
- provide a means of controlling system fluid expansion and contraction,
- provide a means of controlling a system’s pressure, and
- provide a means of removing dissolved gasses from the system by venting the vapor space of the pressurizer.
Construction
A dynamic pressurizer is constructed from a tank equipped with a heat source such as electric heaters at its base, a source of cool water, and a spray nozzle. A spray nozzle is a device located in the top of the pressurizer that is used to atomize the incoming water.
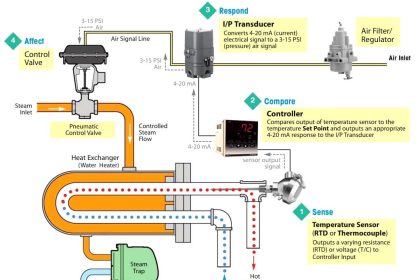
A dynamic pressurizer must be connected in the system to allow a differential pressure to exist across it. The bottom connection, also called the surge line, is the lower of the two pressure lines. The top connection, referred to as the spray line, is the higher pressure line. Differential pressure is obtained by connecting the pressurizer to the suction and discharge sides of the pump servicing the particular system. Specifically, the surge (bottom connection) is connected to the pump’s suction side; the spray line (top connection) is connected to the pump’s discharge side. A basic pressurizer is illustrated in Figure 15.
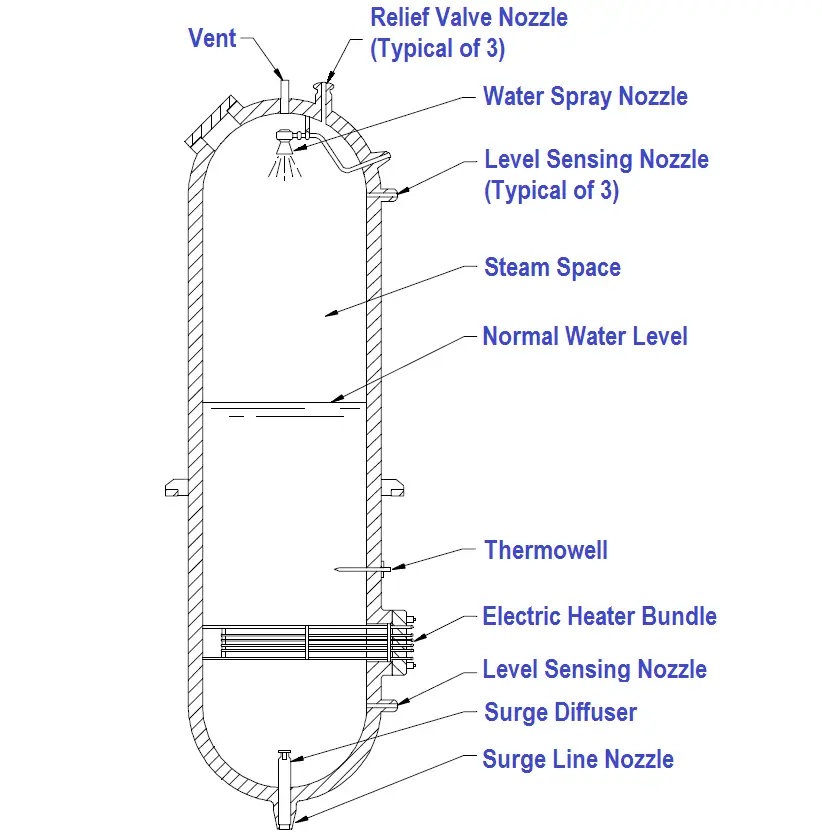
Figure 15 Basic Pressurizer
The hemispherical top and bottom heads are usually constructed of carbon steel, with austenitic stainless steel cladding on all surfaces exposed to the reactor system water.
The pressurizer can be activated in two ways. Partially filling the pressurizer with system water is the first. After the water reaches a predetermined level, the heaters are engaged to increase water temperature. When the water reaches saturation temperature, it begins to boil. Boiling water fills the void above the water level, creating a saturated environment of water and steam. The other method involves filling the pressurizer completely, heating the water to the desired temperature, then partially draining the water and steam mixture to create a steam void at the top of the vessel.
Water temperature determines the amount of pressure developed in the steam space, and the greater the amount of time the heaters are engaged, the hotter the environment becomes. The hotter the environment, the greater the amount of pressure.
Installing a control valve in the spray line makes it possible to admit cooler water from the top of the pressurizer through the spray nozzle. Adding cooler water condenses the steam bubble, lowers the existing water temperature, and reduces the amount of system pressure.
Operation
The level of water within a pressurizer is directly dependant upon the temperature, and thus the density, of the water in the system to which the pressurizer is connected. An increase in system temperature causes the density of the water to decrease. This decreased density causes the water to expand, causing the level of water to increase in the vessel. The increased level of water in a pressurizer is referred to as an insurge. An insurge compresses the vapor space, which in turn causes the system pressure to rise. This results in slightly superheated steam in contact with the subcooled pressurizer liquid. The superheated steam transfers heat to the liquid and to the pressurizer walls. This re-establishes and maintains the saturated condition.
A decrease in system temperature causes the density to increase which causes the system water volume to contract. The contraction (drop) in pressurizer water level and increase in vapor space is referred to as an outsurge. The increase in vapor space causes the pressure to drop, flashing the heated water volume and creating more steam. The increased amount of steam re-establishes the saturated state. Flashing continues until the decrease in water level ceases and saturated conditions are restored at a somewhat lower pressure.
In each case, the final conditions place the pressurizer level at a new value. The system pressure remains at approximately its previous value, with relatively small pressure variations during the level change, provided that the level changes are not too extreme.
In actual application, relying on saturation to handle all variations in pressure is not practical. In conditions where the system water is surging into the pressurizer faster than the pressurizer can accommodate for example, additional control is obtained by activating the spray. This spray causes the steam to condense more rapidly, thereby controlling the magnitude of the pressure rise.
When a large outsurge occurs, the level can drop rapidly and the water cannot flash to steam fast enough. This results in a pressure drop. The installed heaters add energy to the water and cause it to flash to steam faster, thereby reducing the pressure drop. The heaters can also be left on to re-establish the original saturation temperature and pressure. In certain designs, pressurizer heaters are energized continuously to make up for heat losses to the environment.
The pressurizer’s heater and spray capabilities are designed to compensate for the expected surge volume. The surge volume is the volume that accommodates the expansion and contraction of the system, and is designed to be typical of normal pressurizer performance. Plant transients may result in larger than normal insurges and outsurges. When the surge volume is exceeded, the pressurizer may fail to maintain pressure within normal operating pressures.
Pressurizer operation, including spray and heater operation, is usually automatically controlled. Monitoring is required in the event the control features fail, because the effect on the system could be disastrous without operator action.