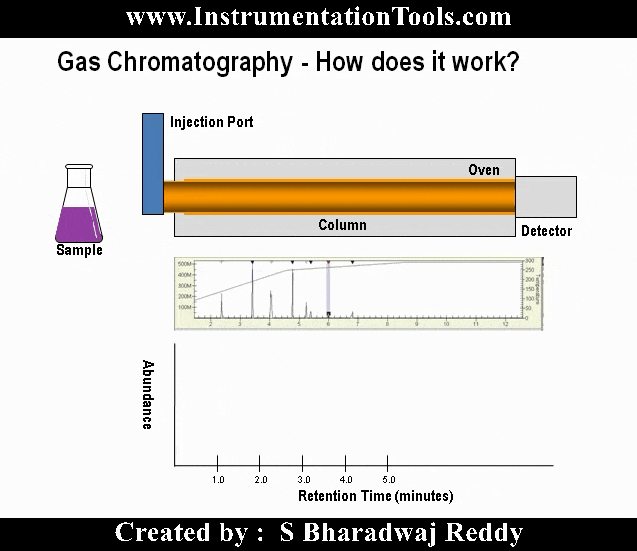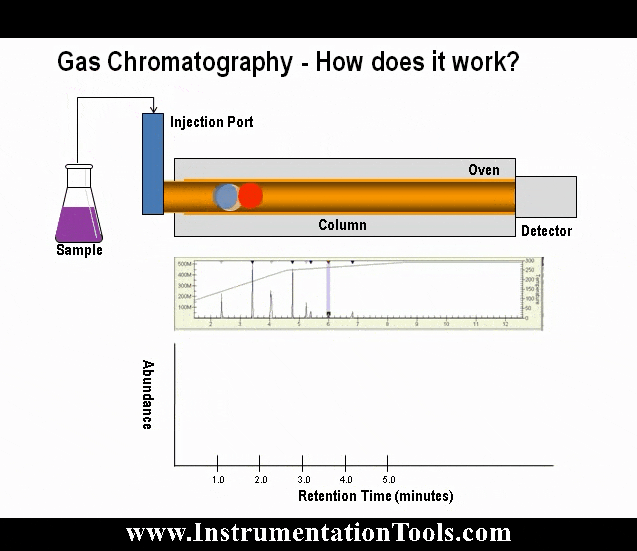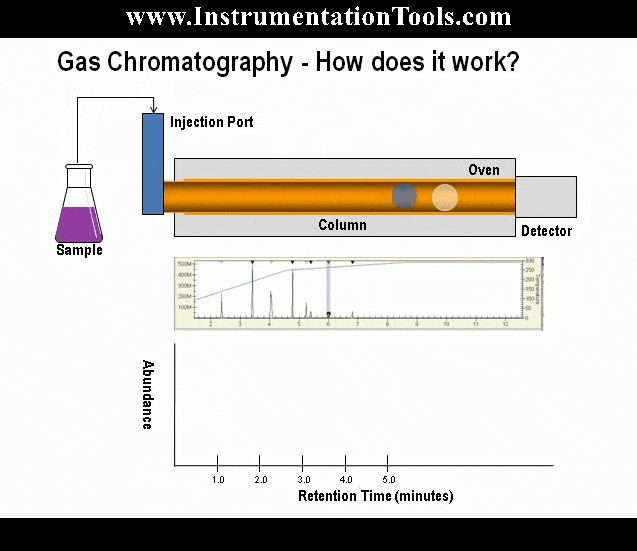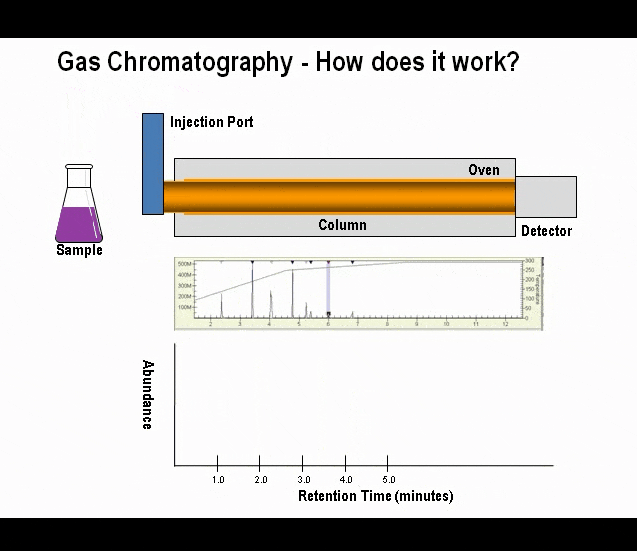
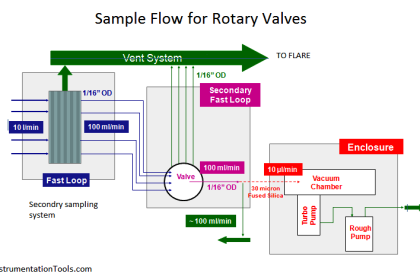
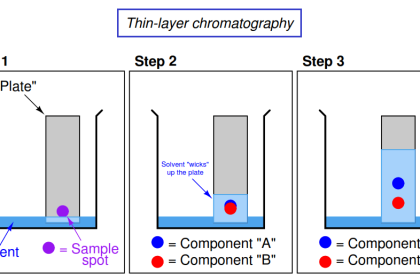
Non-dispersive analysis, while newer in discovery than dispersive analysis (Isaac Newton’s 17th century prism), has actually seen far earlier application as continuous process analyzers. The basic design was developed during the years 1937-1938 by Dr. Luft and Dr. Lehrer in the laboratories of the German chemical company I.G. Farbenindustrie. By the end of World War II, over four hundred of these innovative instruments were in service in German chemical plants. Unlike most industrial analyzer technologies which are nothing more than adaptations of laboratory tests previously used by chemists to take manual measurements of substances, the invention of the first non-dispersive process gas analyzer embodied a wholly new analytical technique.
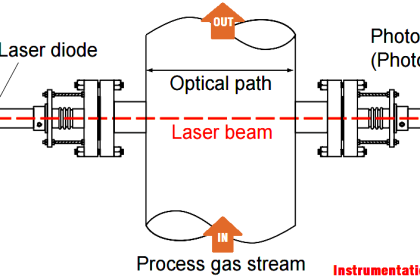
Industrial non-dispersive analyzers typically use either infrared or ultraviolet light sources, because most substances of interest absorb wavelengths in those regions rather than in the visible light spectrum. Non-dispersive spectroscopy using infrared light is usually abbreviated NDIR, while non-dispersive spectroscopy using ultraviolet light is abbreviated NDUV and non-dispersive spectroscopy using visible light is abbreviated NDVIS. Historically, NDIR is the more prevalent of the three technologies. Also, gas analysis is the more common application of non-dispersive spectroscopy in industry, as opposed to liquid analysis, which is why all the examples in this portion of the book assume the analysis of a process gas.
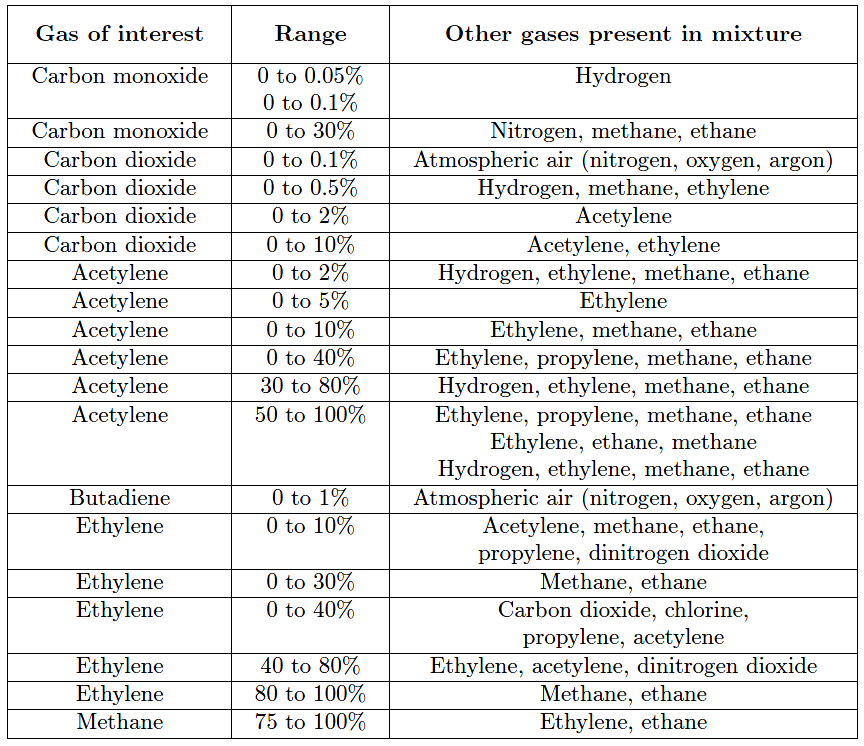
A partial listing of NDIR gas analysis applications at the I.G. Farben synthetic rubber facility in H¨uls, Germany at the conclusion of World War II is shown here. Note the impressive diversity of ranges and gases of interest measured by NDIR analyzers at this time in history, less than ten years following the invention of the technique:
At a different I.G. Farben facility (in Uerdingen, Germany), an NDIR instrument was used as a safety gas detector for carbon monoxide (0 to 0.1% concentration) in open air. This was in a process area where high concentrations of carbon monoxide gas existed in the lines, and where a leak in a process line or valve posed a considerable safety hazard to personnel.
The challenge of any analytical measurement technology is how to achieve selectivity, where the analyzing instrument responds to the concentration of just one substance (one “species”) and to no other substance(s) in the mixture. If the substance of interest exhibits some unique physical property we can readily measure with sensors, the selectivity problem is easy to solve: just measure that one property exclusively, and no other substance will interfere.
In the case of absorption spectrometers such as non-dispersive analyzers, the challenge is to selectively measure the concentration of certain light-absorbing substances amidst the presence of other substances also absorbing certain wavelengths of light. If the substance of interest is the only substance present in the mixture capable of absorbing light, selectivity is guaranteed. However, most applications in industry are not this easy, with the mixture containing other light-absorbing substances besides the one of interest. Some of these substances may absorb completely different wavelengths of light, while others may have absorption bands overlapping the absorption bands of the substance of interest (i.e. the interfering substances absorb some of the same wavelengths of light absorbed by the substance of interest, in addition to absorbing some unique wavelengths of their own).
Dispersive spectrographs achieve selectivity by “disassembling” the spectrum into individual wavelengths and measuring them one by one, but a non-dispersive analyzer must somehow distinguish different spectral responses without this “disassembly” of wavelengths. The bulk of this section is devoted to a discussion of exactly how selectivity is accomplished using the NDIR technique.