The minimum concentration of a flammable gas in air capable of igniting is called the Lower Explosive Limit, or LEL.
This limit varies with the type of gas and with the oxygen concentration of the air in which the flammable gas is mixed.
Sensors designed to detect the dangerous presence of combustible gases are therefore called “LEL sensors.”
LEL monitors are used whenever there is a high probability of explosive gases present in the air.
These areas are referred to as classified areas in industry, and are precisely defined for safety engineering purposes.
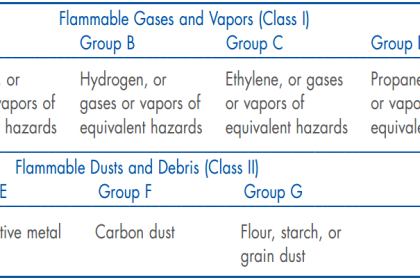
Classified areas harboring explosive gases or vapors are deemed Class I areas, with different “Group” categories delineating the specific gas or vapor types involved.
Gases and vapors are not the only substances with the potential to explode in sufficient concentration.
Certain dusts (such as grain) and fibers (such as cotton) may also present explosion hazards if present in sufficient quantity.
Unfortunately, the majority of analytical technologies used to monitor lower explosive limits for safety purposes only function with gases and vapors (Class I), not dusts or fibers (Class II and Class III, respectively).
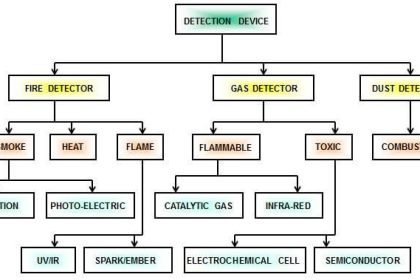
Popular sensor technologies used to detect the presence of combustibles in air include the following:
Catalytic bead and thermocouple sensors both function on the principle of heat generated during combustion.
Air potentially containing a concentration of flammable gases or vapors passes near a heated element, and any combustion occurring at that point will cause the local temperature to immediately rise.
These sensors must be designed in such a way they will not initiate an explosion, but merely combust the sample in a safe and measurable manner.
Like micro fuel cell oxygen sensors, these sensors may be manufactured in sufficiently small and rugged packages to enable their use as portable LEL sensors.
Infrared analyzers exploit the phenomenon of infrared (IR) light absorption by certain types of flammable gases and vapors.
A beam of infrared light passed across a sample of air will diminish in intensity if significant concentrations of the combustible substance exist in that sample.
Measuring this attenuation provides an indirect measurement of explosive potential.
A major disadvantage of this technique is that many non-flammable gases and vapors also absorb IR light, including carbon dioxide and water vapor.
In order to successfully reject these non-flammable substances, the analyzer must use very specific wavelengths of IR light, tuned to the specific substances of interest (and/or wavelengths tuned specifically to the substances of non-interest, as a compensating reference signal for the wavelengths captured by both the substances of interest and the substances of non-interest).
Flame ionization sensors work on the same principle as FIDs for chromatographs: a non-ionizing flame (usually fueled by hydrogen gas) will generate detectable ions only in the presence of air samples containing an ionizing fuel (such as a hydrocarbon gas). Of course, this form of LEL sensor is useless to detect hydrogen gas.
Also Read : Importance of LEL in F&G