As more and more electronic devices use batteries nowadays instead of plugged power supply for use, it matters the most as to which type of battery material is used. In the world of batteries, the most used one is Lithium.
Literally, most electronic devices starting from a simple smartwatch to a mobile phone and other larger electronic equipment use Lithium-Ion battery for use. If a battery is used, then you know that charge and discharge is an important part of it.
Many people ignore the general principles related to lithium-ion battery charging, but it is not a good practice. In this post, we will see the concept related to lithium-ion battery charging systems.
Why is lithium used in batteries?

Before venturing into the topic, let us first see why is lithium used mostly in batteries. The weight of lithium is lower than other minerals like lead, nickel, and cadmium. Weight matters the most as heavy batteries are not attractive to consumers.
The energy density of lithium is very high and among rechargeable minerals, lithium performs the best (means it can charge quickly and discharge after prolonged use and not quickly).
The atomic properties of lithium are such that power flows through it very easily. This is also one of the main reasons why lithium batteries are the most used in electric vehicles nowadays.
How does a lithium-ion battery work?
A battery has two terminals – positive (cathode) and negative (anode). Current flows through these two terminals to the load, which enables the load to do its function. So, how does a battery produce current if there is no external power supply given to it? This all is possible through chemical reactions happening inside the battery.
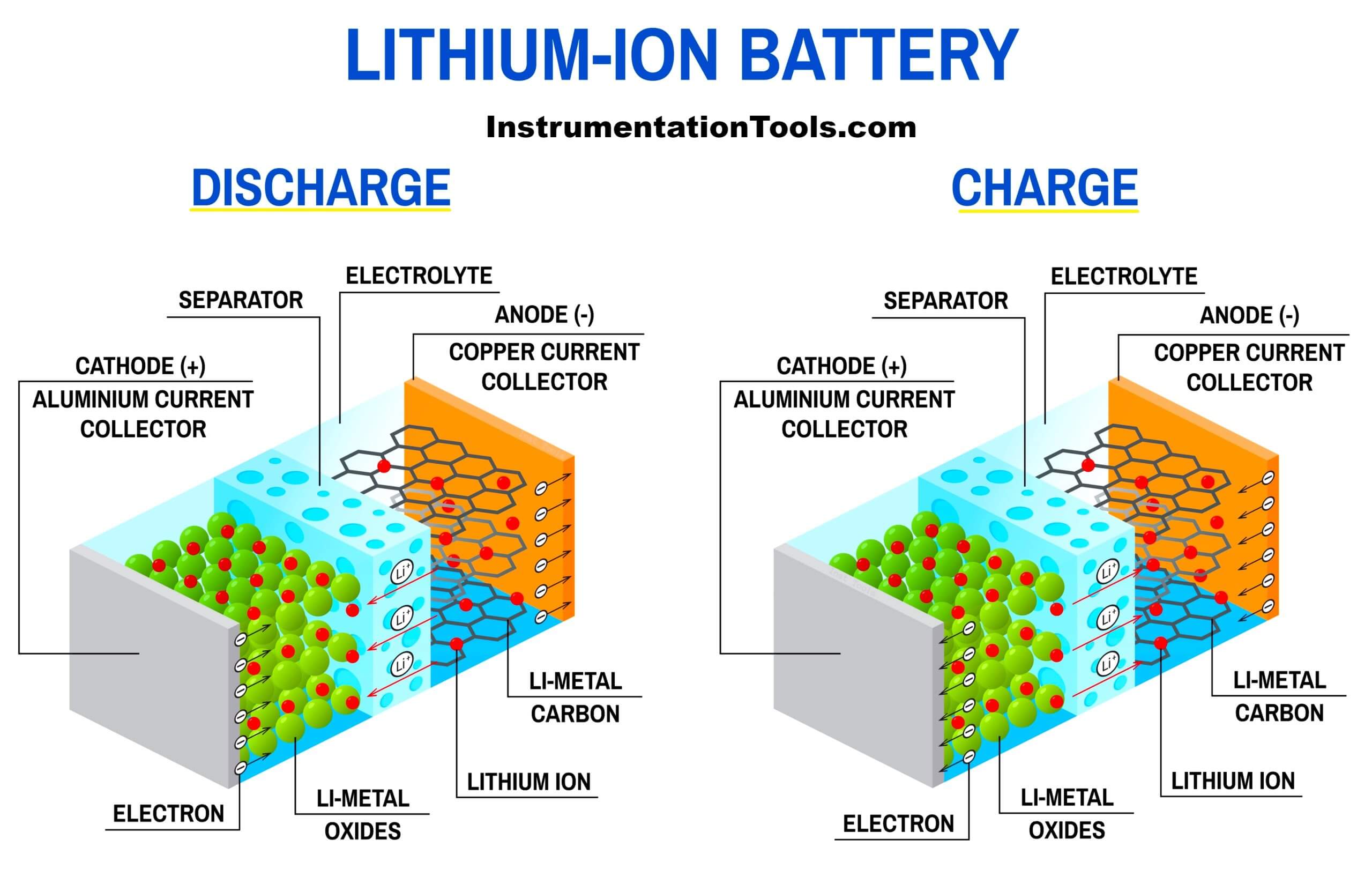
In our discussion, lithium is the main mineral present inside the battery. It is stored in the anode side between layers of carbon graphite. Its main purpose is to give away their free ions and lithium does this role very smoothly.
When the ions from lithium merge with positive atoms (mostly cobalt on the positive side), electricity flow happens. This process is called discharging because as the load requires electricity, the lithium does the function by releasing its ions to the cathode.
During charging mode, the battery requires its ions back and this is done by the cathode. The cathode takes the power supply and provides its ions to the anode. When the anode gets back the ions, it is charged again. This is the most basic and simple concept of charging and discharging.
The whole charging/discharging process is defined as a cycle. The number of cycles that your battery can perform varies depending on the manufacturing process, the chemical components, and the actual usage. The capacity of a rechargeable battery is measured in Ah.
Lithium-ion Battery Charging System
The main considerations for lithium-ion battery charging are mentioned below.
- Constant current to constant voltage method
- Temperature Check
- C Rate
- Overcharging
Constant current to constant voltage method
Charging a lithium-ion battery takes the general principle of constant current constant voltage (CCCV). A constant current is applied to the battery first, which takes to the end-of-charge voltage level. This is the voltage after which the current should not be increased.
Once the level is reached, the current level starts to decrease. This is the charging process. When the current decreases and becomes very low, then it means the charging has been finished. It is to be noted that as the current decreases, the voltage is maintained at the end of the charge voltage level.
This voltage then does the further job of charging the battery till it is not full. So, this voltage is a very important factor in deciding battery performance. Lowering this voltage lowers battery performance.
Temperature Check
Because electricity generates heat during use, the same can be related here during battery charging. Nowadays, as fast charging is a demand from consumers, heat dissipation can be an important factor in deciding battery performance.
So, a battery unit always has a heat dissipation system for controlling the heat generated and dissipating it if it goes to an excess level.
C Rate
This rate is termed as charging and discharging rate of the battery. It is determined by C/N rating. Charge and discharge currents are typically expressed in fractions or multiples of the C rate: A C charge/discharge refers to charging or discharging a battery in one hour. N denotes the number.
For example, a C/2 denotes that the battery takes 2 hours to charge and discharge, whereas a 2C denotes that the battery takes 30 minutes to charge and discharge. So, as the number increases (means C/2 – C/3 – C/6 – C/10 and so on) the time too increases for charging and discharging.
At high charging rates, the lithium ions cannot properly penetrate the electrode and instead accumulate on its surface, which decreases the life of the battery. So, the C rate is a very crucial factor for deciding battery performance.
This is because a very poor C rate means the battery will not age more and a very high C rate means the time is very quick and heat dissipation too will increase at later stages.
Overcharging
If the battery is kept for a charge instead of being full, then it means the lithium-ion has started to float. Once floated after a certain period of time, it can either blast it or reduce the life of the battery.
So, nowadays, many smart circuits are integrated with the battery unit which informs the user if the battery is full or just cuts off its internal supply to the battery, preventing it from overcharging. This avoids heat generation and extends the battery life too.
These are some of the most common considerations during charging a lithium-ion battery. In this way, we saw the concept of a lithium-ion battery charging system.
If you liked this article, then please subscribe to our YouTube Channel for Electrical, Electronics, Instrumentation, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Top 100 Power Systems Projects
- Why Neutral and Earth Separated
- Difference between UPS and Stabilizer?
- Difference Between AA and AAA Batteries
- Valve Regulated Lead Acid (VRLA) Batteries