Semiconductors are a very integral part of our lives. Be it a simple smart watch or a big naval ship, each and every part of it uses a semiconductor material in it. They are the most important invention of the electronics industry, which gained a vast boom after their technological development. Without them, it would be virtually impossible to use our daily devices like mobiles and laptops.
Semiconductors are classified in majorly two categories – intrinsic and extrinsic. In this post, we will learn what they are and what the difference between them is.
What is a Semiconductor?
As you know, electrical conduction normally happens in two types of materials – conductor, and insulator. A conductor is a material that allows the flow of electricity and an insulator is a material that blocks the flow of electricity. When electricity is applied to a conductor material like copper, aluminum, steel, etc., it flows through it and the current can then be passed to the next connecting load.
When electricity is applied to an insulator material like powder, glass, plastic, etc., it does not flow through it and the current is blocked from meeting the load. But, between both of them, there is one more type of material that acts as a median between conductor and insulator. It is called a semiconductor. Let us see how it functions.
We all understand that an atom has two components – photon and electron. Photons are positively charged particles, whereas electrons are negatively charged particles. Electrons are the outermost particles present in an atom boundary.
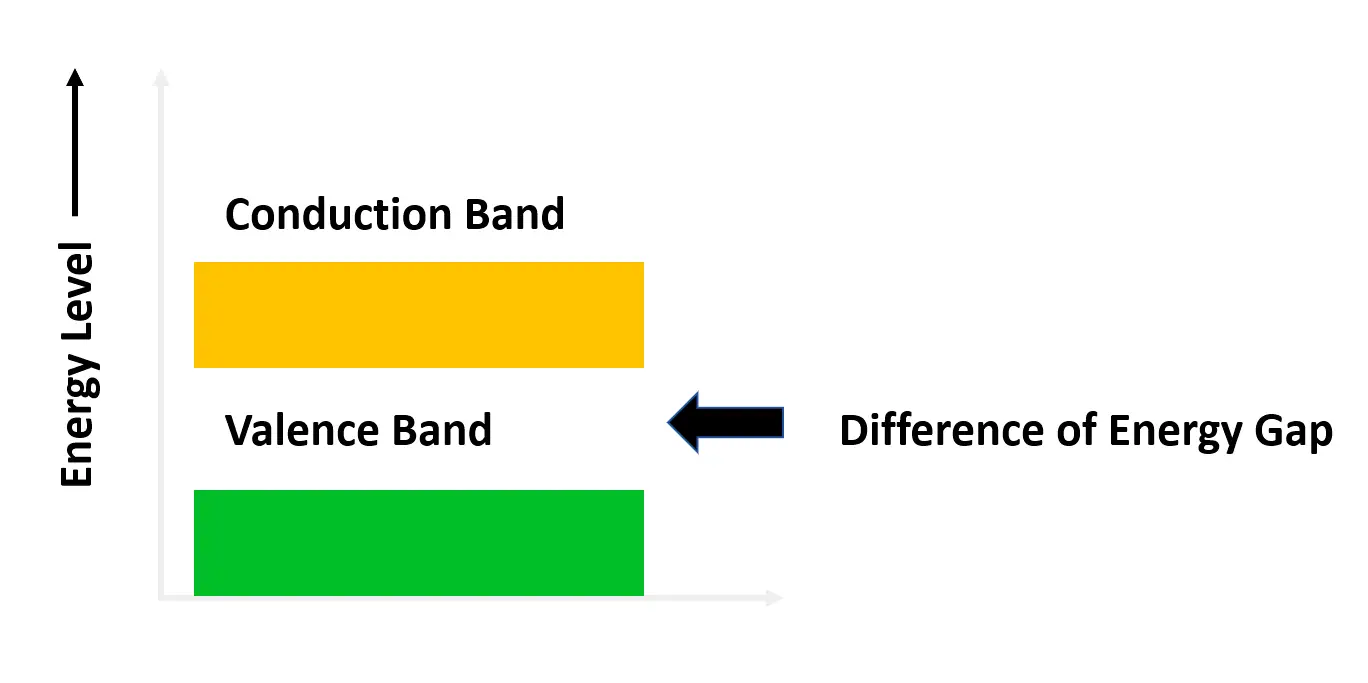
When an electric voltage is applied to the atom, the electrons move out freely from these boundaries due to the thermal energy. Electrons have different energy levels. The electrons with the lowest energy levels come in the valence band and the electrons with the highest energy levels come in the conduction band.
Coming back to our topic, when the electrons move out from their atom to another part, they must pass through a material to reach that higher level of conduction from the valence band. In a conductor, the distance between the valence and conduction band is equal to nil, which allows the easy transfer of electrons to higher energy levels, thus allowing the flow of electricity through them.
In an insulator, the distance between the bands is very high, which does not allow the electrons to reach higher energy levels; thus blocking the flow of electricity. But, in a semiconductor, the distance between the bands is equal to a medium level. Some electrons will flow and some will not.
So, a semiconductor can be defined as a material that has the conductivity between a conductor and an insulator. The most used semiconductors are silicon and germanium. Refer to the below image for more understanding.
What is an Intrinsic Semiconductor?
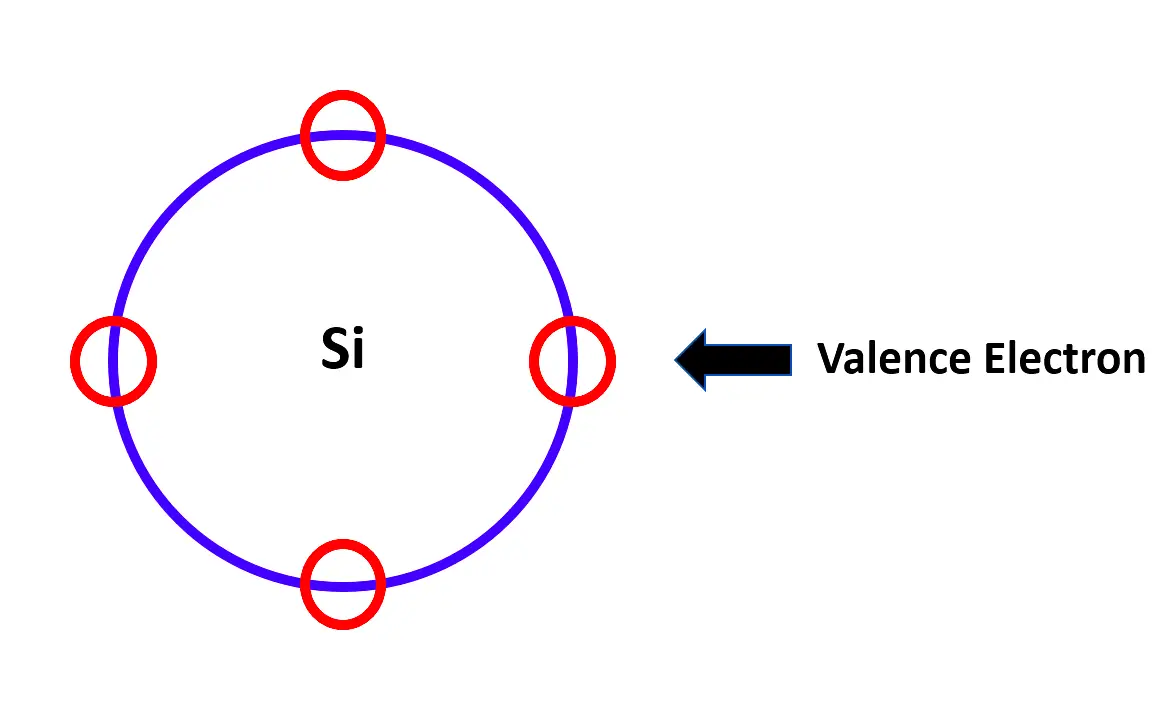
Refer to the below image. As discussed earlier, electrons are present in the outer boundary of an atom. In our case, as we are studying semiconductors, let us take silicon for example. Silicon atom has four valence electrons in its boundary. This is one such atom. There are many such atoms bonded together.
When electrical energy is applied, the electrons start moving away freely from atom to atom, into higher energy levels. If pure silicon atoms are only present in a semiconductor, then it is called an intrinsic semiconductor.
A semiconductor material in its pure form is known as an intrinsic semiconductor. Thus, the intrinsic semiconductors are chemically pure, i.e. they are free from impurities. The number of free electrons is equal to the number of holes in the intrinsic semiconductor.
What is an Extrinsic Semiconductor?
Refer to the same above image for understanding. Now, suppose we add an impurity (atom) with a high number of electrons than the silicon atom. When electrical voltage is applied, extra electrons (electrons left after bonding with impurity atoms ones) too will move into the conduction band. This makes the material negatively charged.
Now, suppose we add an impurity (atom) with less number of electrons than a silicon atom. When electrical voltage is applied, a deficiency of electrons (electrons left without bonding with impurity atoms ones) creates holes, which will move into the conduction band. This makes the material positively charged. This process is called doping.
When a small amount of chemical impurity is added to an intrinsic semiconductor, then the resulting semiconductor material is known as an extrinsic semiconductor.
Difference between Intrinsic and Extrinsic Semiconductors
The main differences between intrinsic and extrinsic semiconductors are as follows.
- Due to the addition of impurities, the conductivity rate of extrinsic semiconductors is very good as compared to intrinsic semiconductors. A controlled manner of conductivity is possible in the case of extrinsic ones.
- The conductivity of intrinsic depends purely on temperature, as it will allow movement of particles; but in extrinsic one, apart from temperature, conductivity is also dependent on doping done.
- The number of holes and electrons are not equal in an extrinsic one, as compared to an intrinsic one where the number of holes and electrons are the same.
- An extrinsic semiconductor can be classified as p-type and n-type ones, but intrinsic semiconductor does not have any classification.
- Intrinsic semiconductors have a low operating temperature, but the operating temperature for extrinsic semiconductors is high.
In this way, we understand the difference between intrinsic and extrinsic semiconductors.