An alternative method of measuring gas concentration is based on absorption of infrared (IR) radiation at certain wavelengths as it passes through a volume of gas. Devices using this technology have a light source and a light detector and measure the light intensity at two specific wavelengths, one at an absorption (active) wavelength and one outside of the absorption (reference) wavelength. If a volume of gas passes between the source and detector, the amount of light in the active wavelength falling on the detector is reduced, while the amount of light in the reference wavelength remains unchanged. Much like the catalytic detectors, the gas concentration is determined from the relative difference between the two signals.
There are several key advantages to using IR-based detectors:
• Immune to all chemical poisons
• Does not need oxygen or air to detect gas
• Can work in continuous exposure gas environments
• Fail-to-safe technology
• Internal compensation virtually eliminates span drift
IR-based detectors can be either single-point or open path devices and, with the sophisticated optical designs currently being used, are factory calibrated and virtually maintenance free. This is particularly desirable when sensors must be located in inaccessible areas and cannot be easily calibrated on a periodic basis. Maintenance of IR detectors is typically limited to periodic cleaning of the optical windows and reflectors to ensure dependable performance. The current availability of reliable, low cost electronics and solid state IR detectors has reduced costs and made the technology feasible for many commercial applications. However, IR detectors cannot be used for the detection of hydrogen and certain other gases for which the catalytic method is suitable.
Infrared gas detection is based on the ability of some gases to absorb IR radiation. It is well known that almost all hydrocarbons (HC) absorb IR at approximately 3.4 µm and at this region H2O and CO are not absorbed, making the system immune to humidity and atmospheric changes. It follows therefore that a dedicated spectrometer operating at that wavelength could be used to detect hydrocarbons in air. Such a system would follow the Beer-Lambert Law which states:
T=exp (-A x C x L)
Where:
T is the transmittance of IR
A is the absorption coefficient of the particular gas molecule
C is the concentration of the gas
L is the path length of the beam through the gas
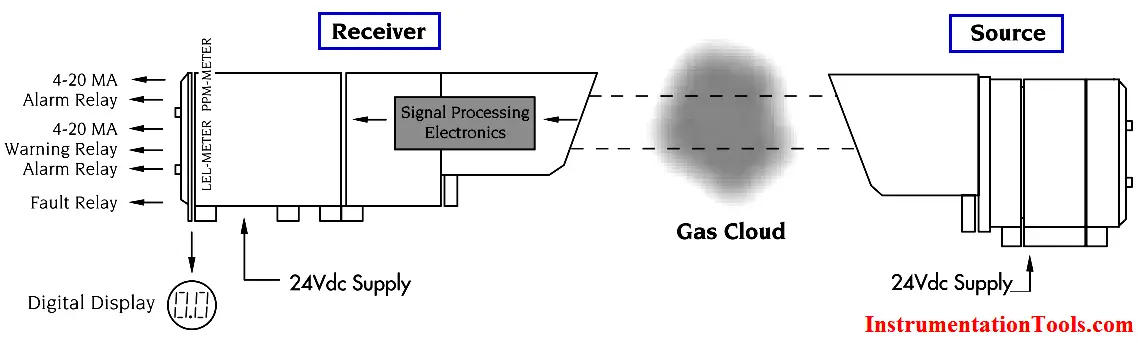
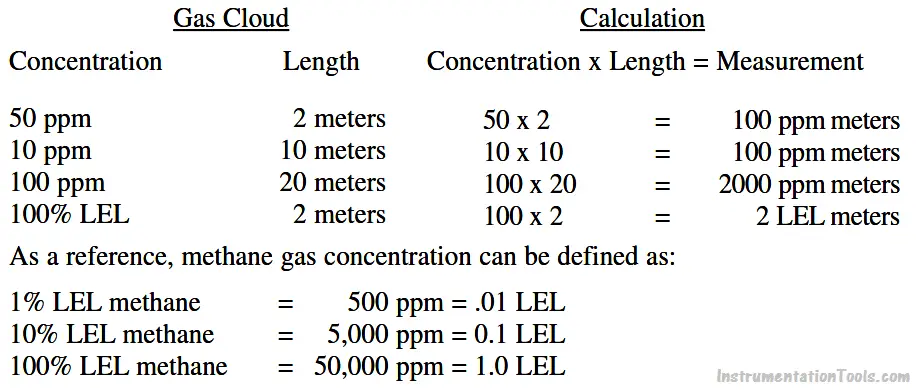
The gas concentration output for open path detectors is expressed in ppm meters (parts per million of combustible gas times the path length in meters: a highly sensitive range for detection of low level leaks) or LEL meters (a hazardous gas level).
Typical readings are as follows:
Open path IR detection offers immunity to poisons, high sensitivity gas leak detection, hazardous level gas detection, low maintenance, easy installation and fail to safe operation. However, it is not an all-encompassing answer to combustible gas detection. It offers an alternative solution to gas detection challenges and should be used in combination with point gas detection due to its limitations in targeting the specific location of gas leaks.












Send me PDF version of that to read for maintenance of gas detectors.
Please, I need a material for the calibration of open path detectors