Gas Chromatography Questions & Answers
1. For the separation of which of the following substances, Gas-solid chromatography is being used?
a) Thermally stable organic components
b) Volatile organic components
c) Thermally stable inorganic components
d) Low molecular weight gaseous species
Answer: d
Explanation: Gas-solid chromatography is used for the separation of low molecular weight gaseous species. Its application is limited because of semi-permanent retention of the analyte.
2. Which of the following is not a feature of carrier gas used in gas chromatography?
a) It must be chemically inert
b) It should be suitable for the detector employed
c) It should not be completely pure
d) It should be cheap
Answer: c
Explanation: It should be highly pure. Further, it should be readily available and non-inflammable.
3. Which of the following is the disadvantage of hydrogen, which can be used as carrier gas in gas chromatography?
a) Dangerous to use
b) Expensive
c) Reduced sensitivity
d) High density
Answer: a
Explanation: Hydrogen is dangerous to use. It has better thermal conductivity and lower density.
4. Which of the following is the disadvantage of helium, which can be used as carrier gas in gas chromatography?
a) Dangerous to use
b) Expensive
c) Reduced sensitivity
d) High density
Answer: b
Explanation: Helium is expensive. Its advantages are that it has low density and it allows greater flow rates.
5. Which of the following is the disadvantage of nitrogen, which can be used as carrier gas in gas chromatography?
a) Dangerous to use
b) Expensive
c) Reduced sensitivity
d) High density
Answer: c
Explanation: Nitrogen has reduced sensitivity. It is still one of the commonly used carrier gas in gas chromatography.
6. Slow injection of large samples leads to band broadening and loss of resolution.
a) True
b) False
Answer: Slow injection of large samples leads to band broadening and loss of resolution. Hence, for desired column efficiency, samples should not be too large.
7. In which of the following methods are liquid samples injected into the column in gas chromatography?
a) Gas-tight syringe
b) Micro-syringe
c) Rotary sample valved
) Solid injection syringes
Answer: b
Explanation: Liquid samples injected into the column in gas chromatography using micro-syringe. Syringes of various capacities are available.
8. What must be done to the solid samples for it to be introduced into the column without using solid injection syringes in gas chromatography?
a) Introduced in hot-zone of the column
b) Dissolved in volatile liquids
c) Introduced using rotary sample valved
) Introduced using sampling loops
Answer: b
Explanation: Solid samples must be dissolved in volatile liquids for introducing it into the column. They can be introduced directly using solid injection syringes.
9. Which of the following is the commonly used support material for the packed column in gas chromatography?
a) Glass
b) Metal
c) Diatomaceous earth
d) Stainless steel
Answer: c
Explanation: Diatomaceous earth is the commonly used support material for the packed column in gas chromatography. The columns could be made of glass or metal.
10. Which of the following is the advantage of straight packed column?
a) It can be packed uniformly
b) It can be repacked easily
c) It is compact
d) It is easier to heat it evenly
Answer: c
Explanation: The advantage of straight column is that it can be repacked easily. It is not compact in size.
11. Which of the following is the disadvantage of coiled or helical shaped packed chromatographic column?
a) It cannot be packed uniformly
b) It cannot be repacked easily
c) It is not compact
d) It is not easy to heat it evenly
Answer: b
Explanation: The disadvantage of coiled or helical shaped packed chromatographic column is that it cannot be repacked easily. It is compact in size and can easily be heated in an even manner.
12. Capillary columns are open tubular columns constructed from which of the following materials?
a) Glass
b) Metal
c) Stainless steel
d) Fused silica
Answer: d
Explanation: Capillary columns are constructed using fused silica. It is a very high purity glass.
13. Sample injection port must be maintained at a temperature at which rapid vapourisation occurs but thermal degradation does not occur.
a) True
b) False
Answer: a
Explanation: Sample injection port must be maintained at a temperature at which rapid vapourization occurs but thermal degradation does not occur. The column is maintained at a different temperature.
14. Which of the following is not a desirable feature of the ovens used in gas chromatography?
a) It must have a fast rate of heating
b) Power consumption should be kept low
c) It must have maximum thermal gradients
d) It should have proper insulation
Answer: c
Explanation: The ovens used in gas chromatography must have maximum thermal gradients. The temperature must be uniform over the whole column.
15. Given below is the block diagram of gas chromatography. Identify the unmarked component.
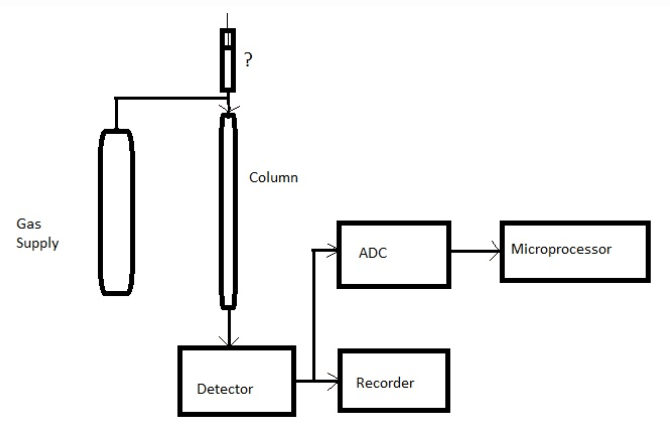
a) Pumping system
b) Pressure regulator
c) Flow regulator
d) Sample injection system
Answer: d
Explanation: The unmarked component is syringe. Hence, the answer is sample injection system. It is for the introduction of sample into the flowing gas stream.