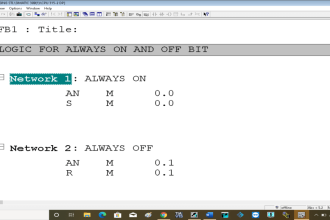
Water has many properties in it. Two of the most generally used properties of water are – adhesion and cohesion. By hearing them, you think that these both are very similar terms. But no, there are vast differences between them.
As water is a very important part of our life, it is necessary to understand how it binds the various molecules in our earth. This serves as a tool in producing materials for our use.
In this article, we will learn the difference between adhesion and cohesion.
What is Adhesion?
Adhesion is used to define a term where water is attracted to other substances. For example, if you have a hydrogen molecule of water and some other molecule of a substance like chlorine, then it will bind together.
Suppose you are joining two wooden materials with fevicol, then this bonding is called as adhesion. That is why, you may also heard of fevicol being categorized into adhesive liquid.
Adhesion is caused by electrostatic or mechanical forces that exist between different substances. From all these, we can also term adhesion as an intramolecular attraction. If you force an adhesive liquid on a surface, then it will spill over it.
What is Cohesion?
Cohesion is used to define a term where water is attracted to only water. For example, if you have two hydrogen molecules of water, then they will bind together. Suppose you are opening a tap of water; then the water which is coming out of it is cohesion. It is due to cohesion only that water molecules are joining together and becoming a flow from the tap.
The cohesive forces are associated with Van der Waals forces and hydrogen bonding that cause liquids such as water to withstand the separation. From all these, we can also term adhesion as an intermolecular attraction.
Difference between Adhesion and Cohesion
Some of the differences are mentioned below.
- Adhesion happens when two dissimilar substances or molecules face the force of attraction between each other; whereas cohesion happens when two similar substances or molecules face the force of attraction between each other.
- Cohesion causes the formation of capillary action, water droplets, and surface tension of a liquid; whereas adhesion causes the liquid to spread on the surface like glue, paint, or cement.
- A very simple example of cohesion is water molecules sticking with each other to form a water droplet, and an easy example of adhesion is the same water droplet being confined at the end of a leaf which prevents it from dropping into the ground (hydrogen molecules of water are binding with molecules of the leaf).
Adhesion and cohesion must be properly studied and co-related when you develop a product which uses water; this helps in efficiently producing it. A force of attraction is an adhesive or cohesive force. Due to these forces, molecules are attracted or repelled by one another. Molecules are attracted to one another by adhesive forces.
An adhesive force is what holds molecules of the same substance together. Furthermore, adhesive and cohesive forces provide much information about biological processes, such as water transport through xylem tubes.
As a general rule, adhesion and cohesion are the properties of molecules of the same substance sticking together, while adhesion refers to molecules of different substances sticking together.