Chlorine (Cl2) gas is a strong-odored, toxic gas used as a biological disinfectant, bleaching agent, and as an oxidizer in many industrial processes. Colorless in low concentrations, it may appear green in color when mixed in very high concentrations with air. Chlorine is highly reactive, presenting a distinct hazard to mucus membranes (eyes, nose, throat, lungs) by creating hypochlorous acid (HOCl) and hydrochloric acid (HCl) upon contact with water:
Cl2 + H2O → HOCl + HCl
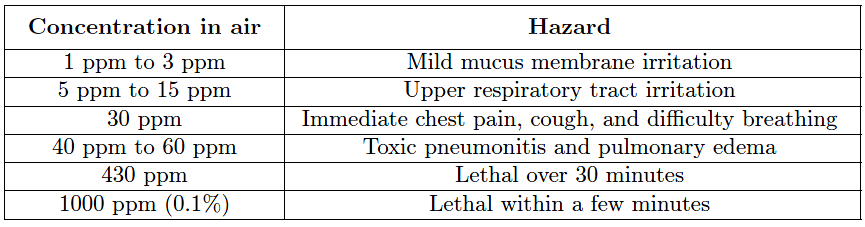
The following table correlates levels of chlorine gas concentration in ambient air with degree of hazard. Note the unit of measurement for chlorine concentration in air – parts per million (ppm). Bear in mind that one part per million is equivalent to just 0.0001 percent:
Water and wastewater treatment operations frequently use chlorine for disinfection of water. Pulp mills use either chlorine or chlorine compounds as a bleaching agent to whiten wood pulp.
Chlorine may be generated on site by the electrolytic decomposition of salt (sodium chloride – NaCl), or delivered in cylindrical pressure vessels in liquid form as shown here at a large wastewater treatment facility: