Chlorine dioxide measurement
Chlorine dioxide (ClO2) is an instable, non-storable, toxic gas with a characteristic scent. The molecule consists of one chlorine atom and two oxygen atoms – represented in the chemical formula ClO2. It is very reactive. To avoid the risk of spontaneous explosions of gaseous chlorine dioxide or concentrated solutions, it is generally handled in dilution with low concentrations. ClO2 is soluble in water, but tends to evaporate quickly. Typically it is prepared on site, for example from hydrochloric acid and sodium chlorite. The procedure provides solutions with approx. 2 g/l ClO2 that can be safely handled and stored for several days.
Image Credits : krohne
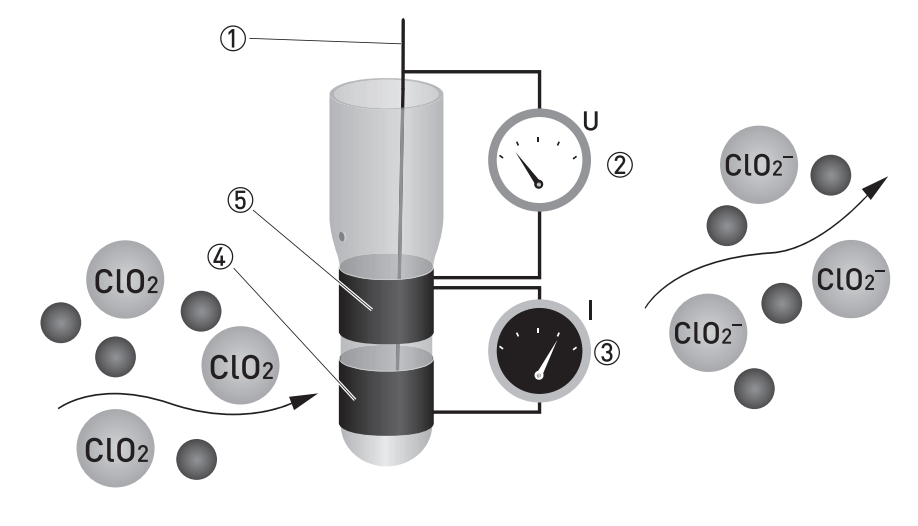
Sensor Parts :
- Reference electrode
- Applied chlorine dioxide specific potential
- Current needed to maintain the constant potential
- Counter electrode
- Measuring electrode
The disinfection effect of ClO2 is due to the transfer of oxygen instead of chlorine, so that no chlorinated byproducts are formed. ClO2 is used as disinfectant against biofilm, bacteria, spores, and viruses. Today it is believed that the molecule´s unpaired electron is transferred to the DNA of the microorganism which cracks and causes cell necrosis. ClO2 has a long-term effect of several days. In contrast to chlorine, the disinfection strength of ClO2 does not depend on pH, and neither does the measurement show a pH influence in the range of pH 6 to pH 9.
ClO2 is measured potentiostatic with measuring and counter electrodes of pure gold and an Ag/AgCl reference. The measurement shows high selectivity towards ClO2. A precise potential is built up between the measuring and the reference electrode. The measuring electrode starts polarising, i.e. ions collect close to the electrode to neutralise the electrical field. ClO2 molecules that hit the surface take a defined portion of the charge with them. The controller measures the potential between measuring and reference electrode and readjusts the charge on the electrode surface. The current needed to maintain a constant potential is directly correlated to the dissolved chlorine dioxide concentration in the measuring medium.
Source : krohne