Direct current is the constant flow of the electric charge from high potential to low potential. A Direct current circuit is a circuit that electric current flows through in one direction only. DC is commonly used in many low voltage applications especially where these are powered by battery.
Nowadays, most electronic circuits require a DC power supply. DC load is the current flow resulting from resistive elements of the load on an output. There is a source of emf with a voltage and measured in units of volts. The most common sources were Battery and Cell.
Electric Cell
An Electric Cell is a power-generating device that converts stored chemical energy into electrical energy. It is the combination of electrodes and electrolytes, where a difference of certain electric potentials is established between the electrodes as a result of the chemical reaction between the electrodes and electrolytes.
The difference in the electric potential between electrodes in an electrical cell depends upon the types of electrolytes and electrodes used.
Some common examples of electric cells are nickel-cadmium cells and lead-acid cells.
Types of Cells
The electrical cell can be divided into the following four categories based on the electrical properties of the cell.
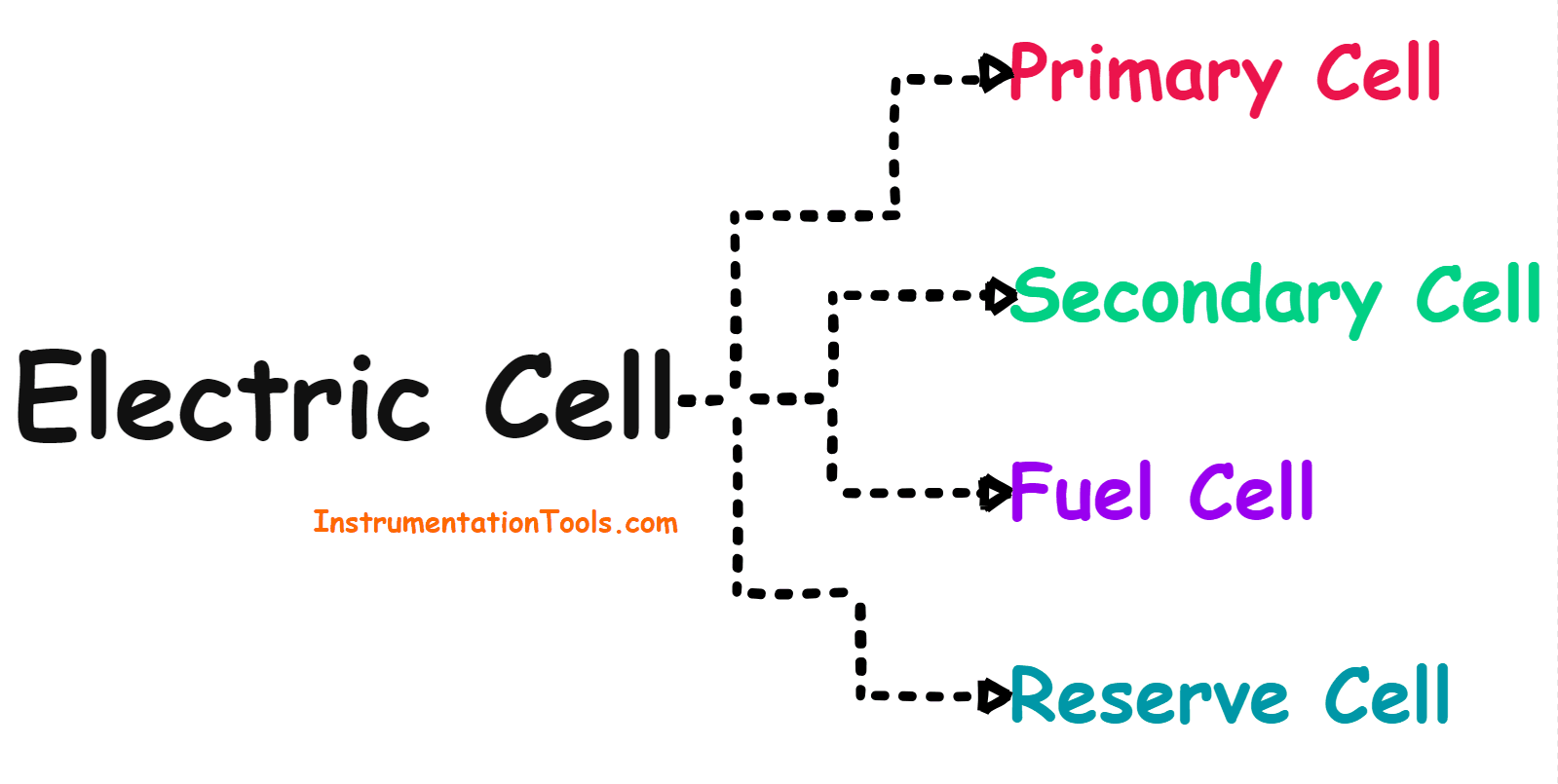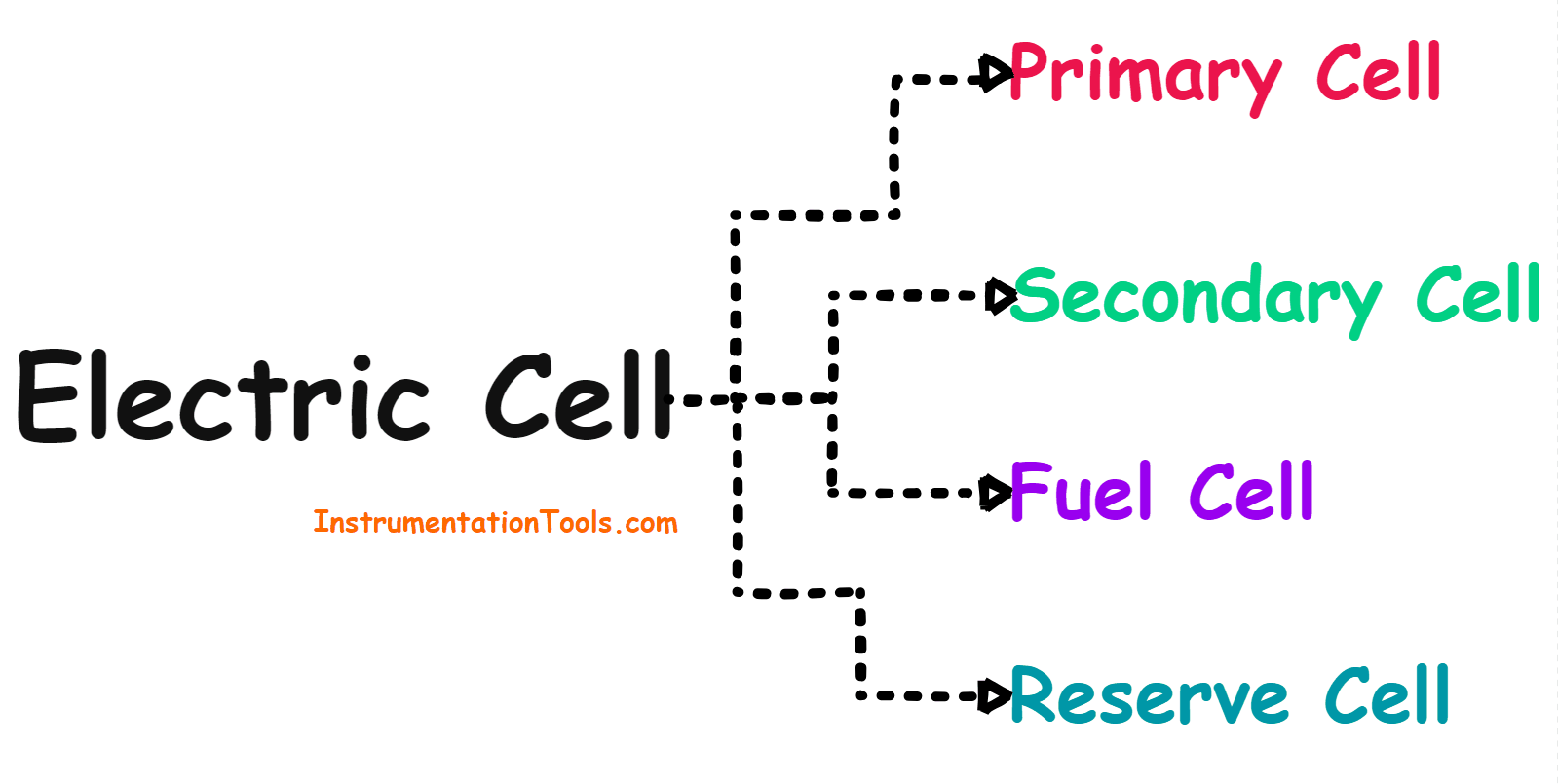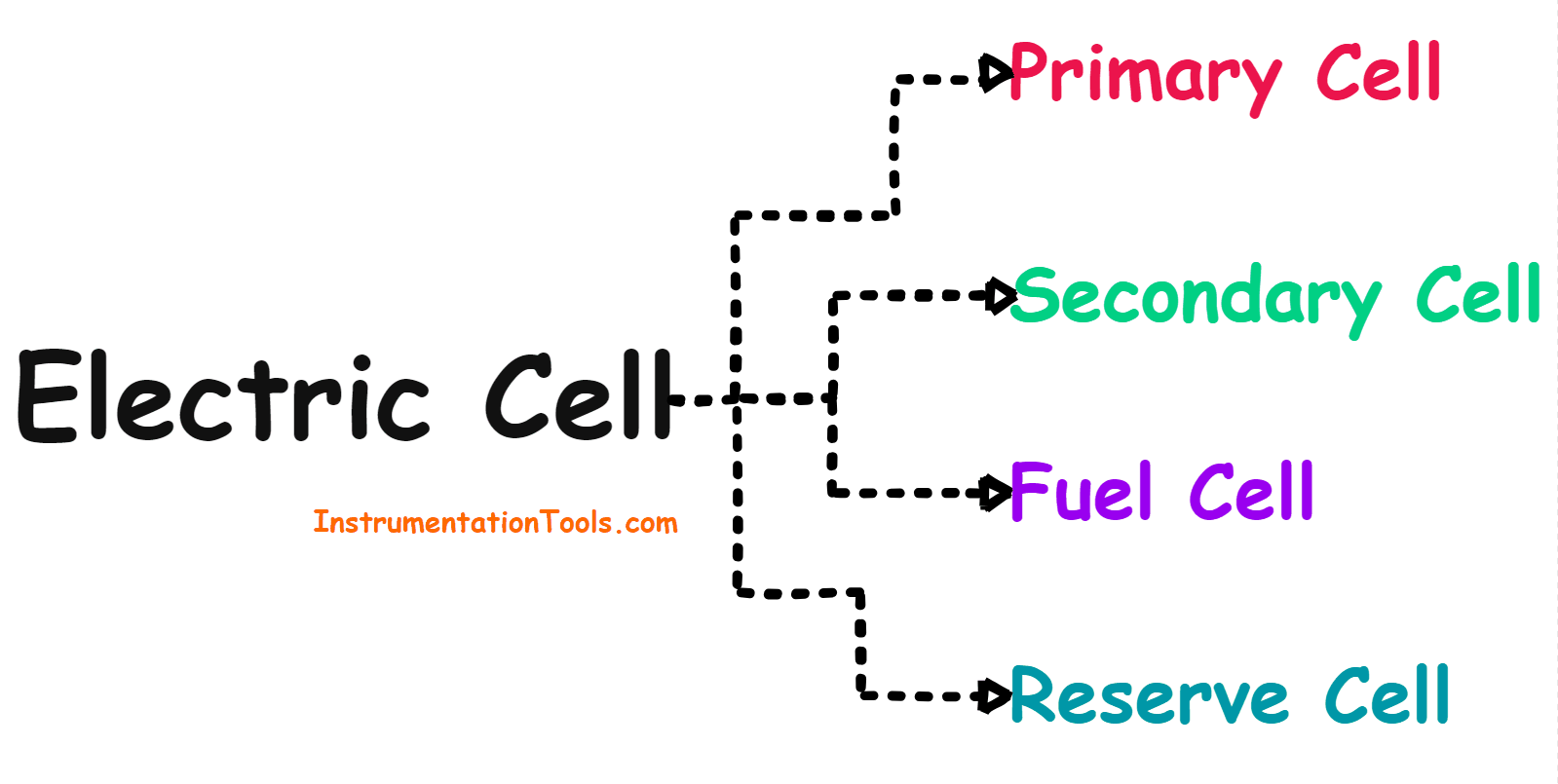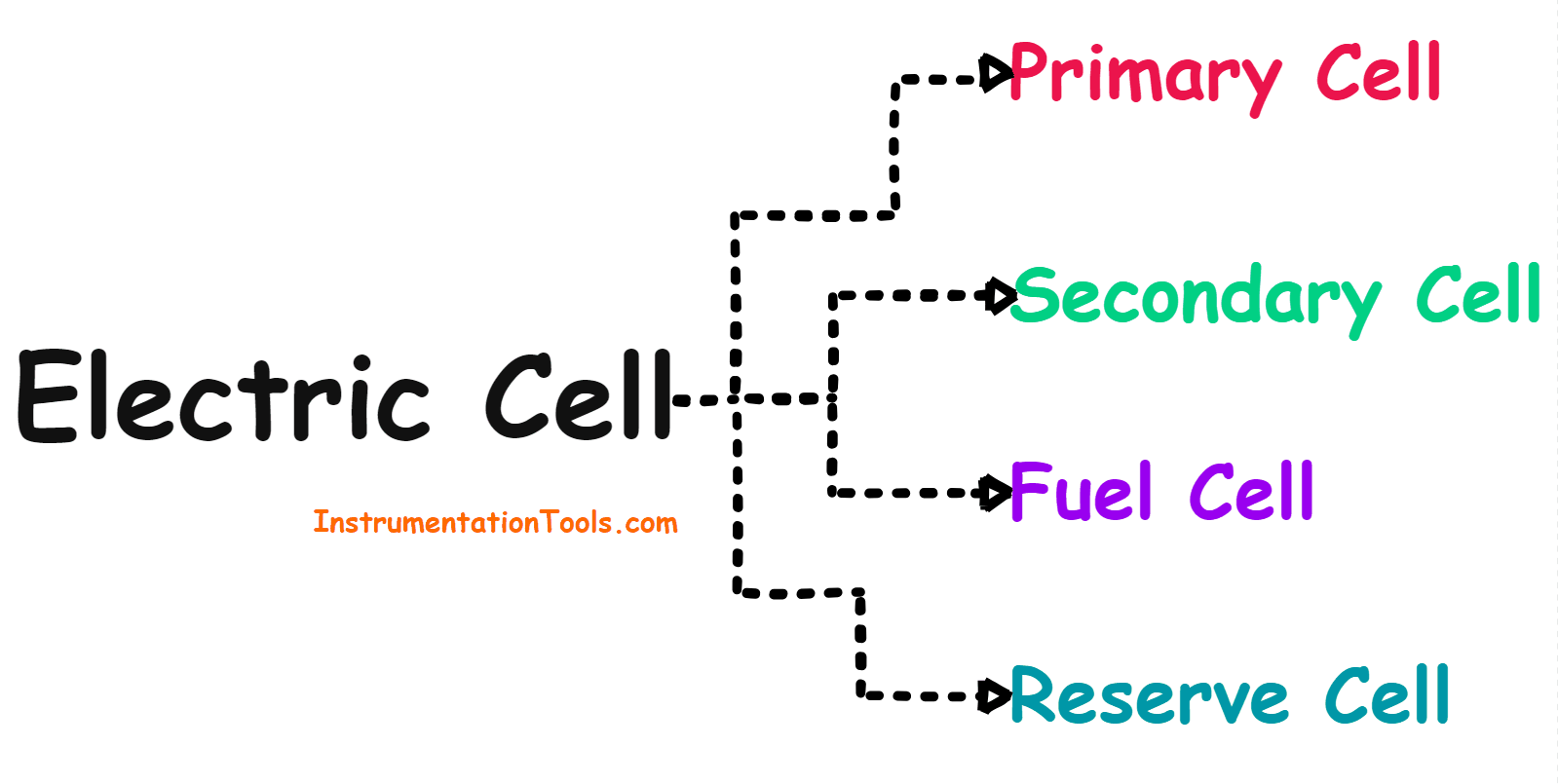
Cells can be divided into the following four categories.
- Primary Cell
- Secondary Cell
- Fuel Cell
- Reserve Cell
Primary Cell
An electrical cell that is powered by an irreversible chemical reaction is called as Primary Cell. Since the primary cell is powered by an irreversible chemical reaction, it cannot be recharged after being used so it can be used for only once and after that, it needs to be decomposed.
Many primary cells contain, electrolytes in solid form absorbed within some absorbent material so these kinds of cells are called Dry Cells. If we recharge it will produce H2 and heat and result in an explosion.
Secondary Cell
The cell which can be recharged after being used is known as the Secondary Cell. These kinds of cells are powered by reversible chemical reactions and the electrodes and electrolytes can be reversed to their original form by applying an external power source after being used.
Secondary cells usually have a high discharge rate compared to the primary cells and can be used with high loads that require good discharge rate performance.
Fuel Cell
A fuel cell is the kind of cell where the chemical energy from a fuel fed into the cell is converted into electrical energy through a chemical reaction with an oxidizing agent.
The fuel that is fed into a fuel cell can be Hydrogen, hydrocarbons, and Natural Gas. A fuel cell can produce electricity as long as the fuel and the oxidizing agent are fed into the fuel cell.
Reserve cell
The type of cell where one of the components of the cell is separated from the rest of the components until activated manually or by some automatic means is called a Reserve cell.
These kinds of cells remain deactivated and the nonfunctional unit is activated manually or by some means like heat, water, etc.
One of the examples is the thermal cell where the electrolyte remains inactive in its solid form until the applied melts the electrolyte to activate the cell.
Battery
A battery is nothing but a combination of cells. Several Cells are combined and connected electrically in series or parallel to form a battery which has two main terminal electrodes one positive and one negative.
The electrical potential difference between the two main electrodes depends upon the number of cells, types of cells, and the types of combination used to form the battery. In electronic circuits, the following symbol is used to determine the symbol of the battery.
Types of Battery
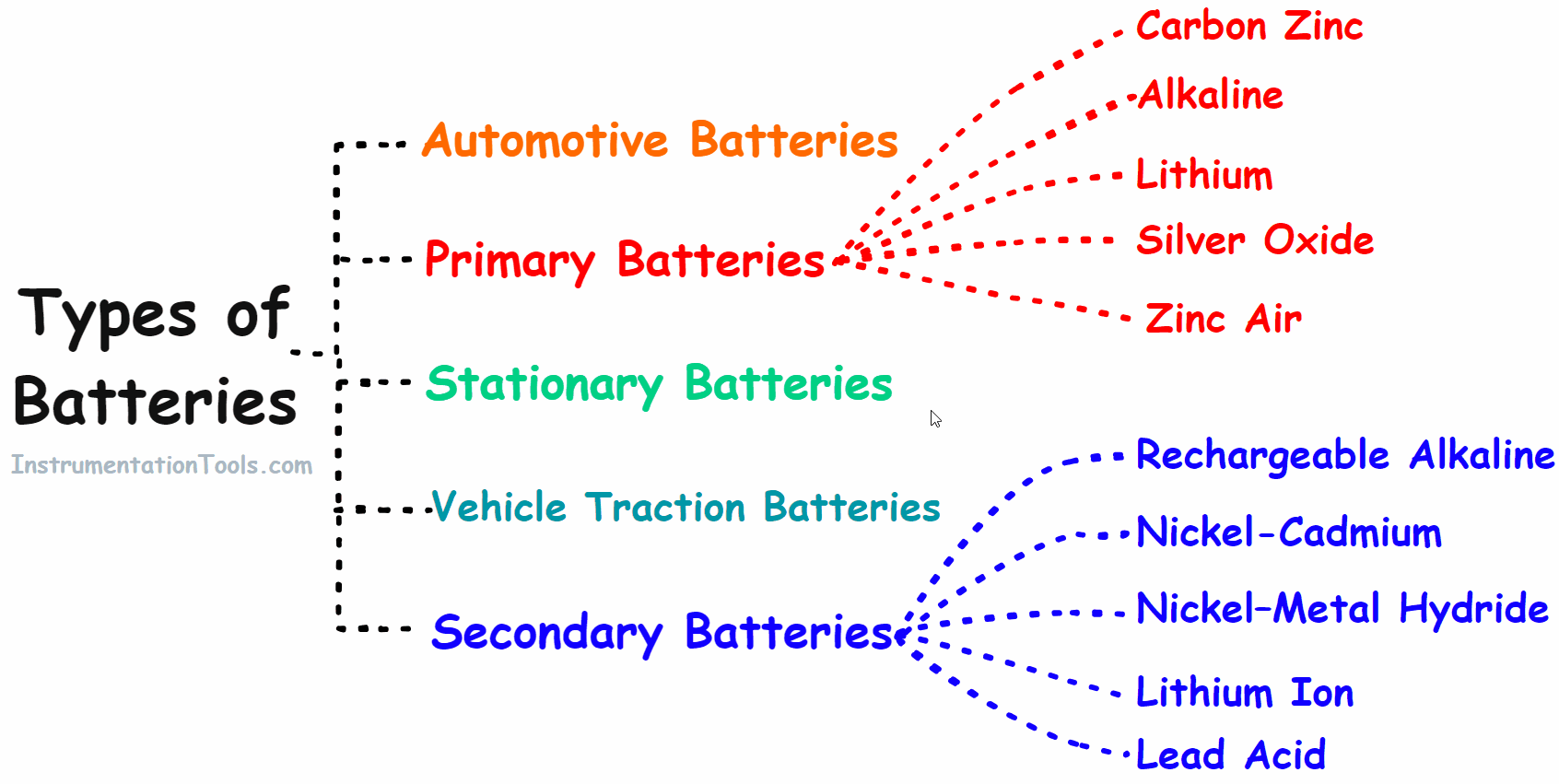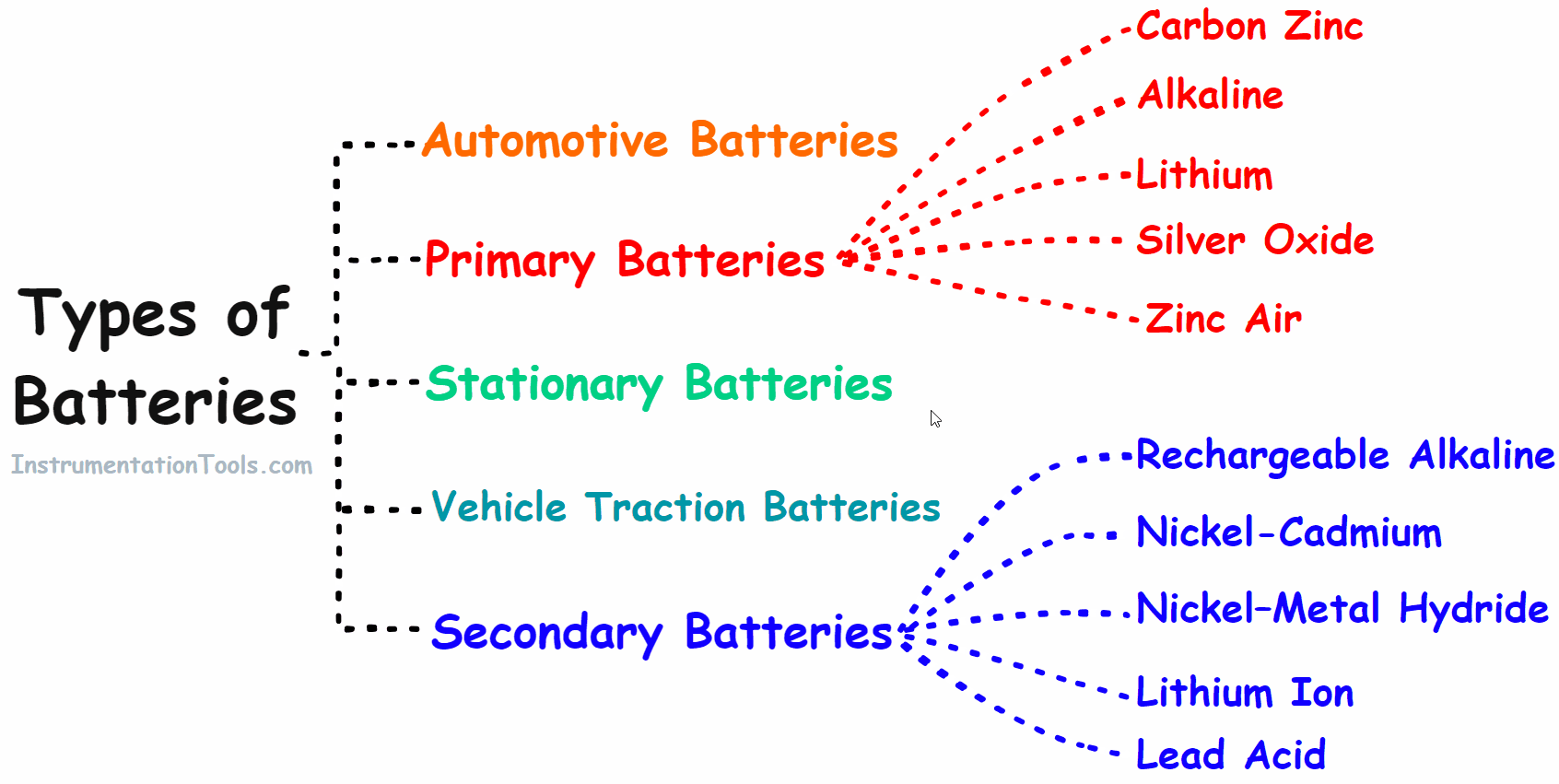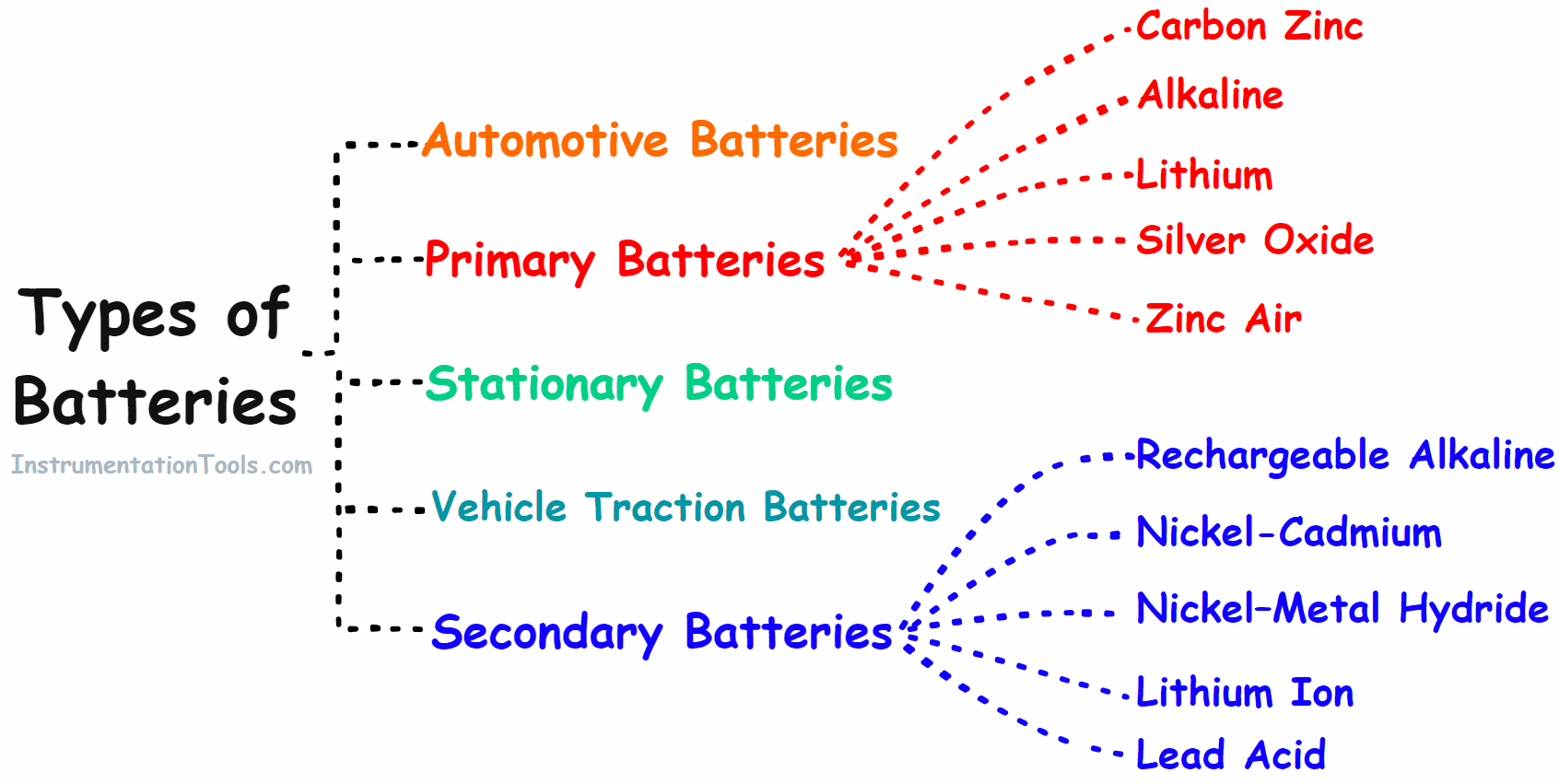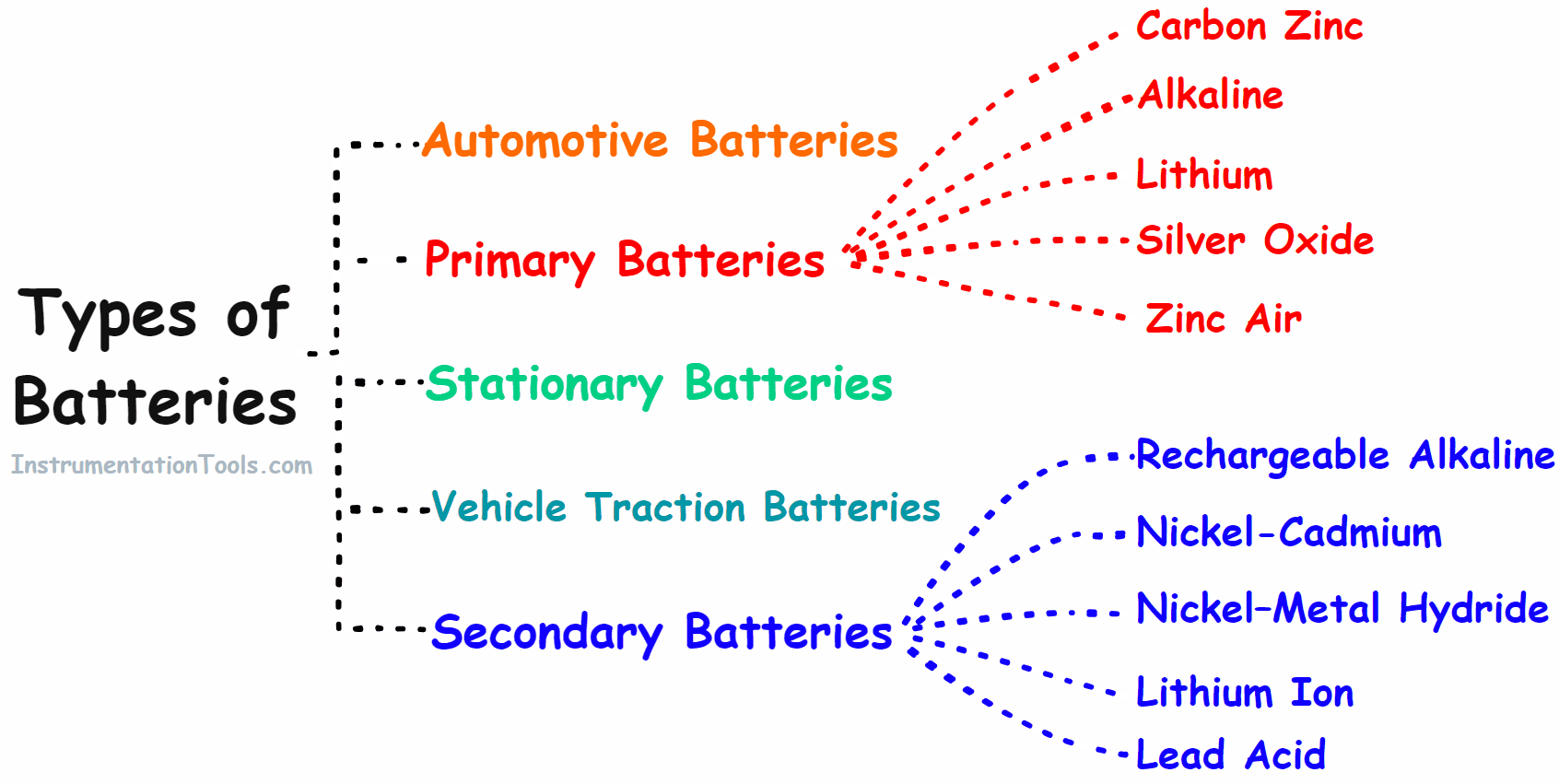
Based on the properties batteries are classified into the following main groups.
- Primary battery
- Secondary battery
- Automotive battery
- Vehicle Traction/Industries Batteries
- Stationary Batteries
Primary Battery
Primary batteries are the type of battery that is made up of primary cells, the energy is inherently present in the cells of Primary Battery.
Some examples are alkaline batteries, lithium cells, and metal-air batteries. Below are some of the primary batteries:
Carbon Zinc Battery:
The lowest cost of the primary cell is the Zinc-acidic manganese dioxide battery. They provide only very low power, but have a good shelf life and are well suited for clocks and remote controls.
Alkaline Battery:
The most commonly used primary cell is the zinc-alkaline manganese dioxide battery. They provide more power per use than Carbon-zinc and secondary batteries and have an excellent shelf life.
Lithium Battery:
Lithium batteries offer performance advantages well beyond the capabilities of conventional aqueous electrolyte battery systems. Their shelf life can be well above 10 years and they will work at very low temperatures.
Lithium batteries are mainly used in small formats because bigger sizes of lithium batteries are a safety concern in consumer applications.
Silver Oxide Battery:
These batteries have a very high energy density but are very expensive due to the high cost of silver. Therefore, silver oxide cells are mainly used in button cell format for watches, calculators, and measuring instruments like vernier calipers.
Zinc Air Battery:
These batteries have become the standard for hearing aid batteries. They have a very long run time because they store only the anode material inside the cell and use the oxygen from the ambient air as a cathode.

Secondary Battery
A secondary battery is a type of battery that is made up of secondary cells. The energy is induced in the chemical of the cells of the secondary battery by applying external energy or sources prior to using it.
Some examples of secondary batteries are lead-acid batteries, nickel-cadmium batteries, and nickel-iron batteries. The secondary batteries can also be classified into the following types based on their usage.
Rechargeable Alkaline:
Secondary alkaline batteries, the lowest-cost rechargeable cells have a long shelf life and are useful for moderate power applications. It has less life than other types of secondary batteries. They have no toxic ingredients and can be disposed of in regular landfills
Nickel-Cadmium:
Secondary nickel cadmium batteries are rugged and reliable. They exhibit a high power -capability, a wide operating temperature range, and a long cycle life, but have a low run time per charge.
They have a self-discharge rate of approximately 30% per month. They contain about 15 % toxic, carcinogenic cadmium and have to be recycled.
Nickel–Metal Hydride:
Secondary Nickel Metal Hydride batteries are an extension of the old-fashioned Nickel-cadmium batteries. This battery provides the same voltage as a Nickel Cadmium battery but offers at least 30% more capacity. They exhibit good high current capability and also have a long life.
Nickel Metal cells contain no toxic cadmium, but they still contain a large amount of nickel oxides and some cobalt, which are known as human poisons and that should be recycled.
Lithium Ion:
Lithium Ion Batteries are the latest breakthrough in rechargeable batteries. They are at least 30% lighter in weight than Nickel Metal batteries and provide at least 30% more capacity. They exhibit good high current capability and have a long lifetime. Its self-discharge rate is also comparatively good.
But these batteries may cause a fire because of overheat. Lithium Ion cells contain no toxic cadmium but they contain cobalt oxides which need to be recycled because it was toxic to humans.
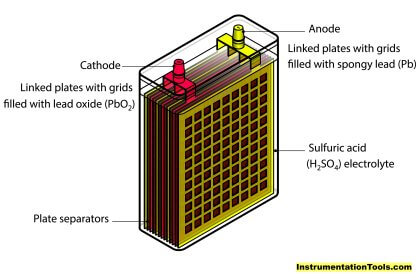
Lead Acid:
Lead Acid batteries are the most popular rechargeable batteries around the world. Its manufacturing process was also economical and reliable. Because of their heaviness, they cannot be used in portable or consumer applications.
Since Lead is a toxic waste, we should not dispose of regular waste. However, recycling lead-acid batteries was very easy and economical. Nearly 90% of the battery lead is being recycled today. Those recycled batteries were used again for the production of new Lead Acid batteries.

Automotive Batteries
These kinds of automotive batteries are used in automobile and portable systems. This battery is used for starting, Lighting, and ignition in automobiles.
Vehicle Traction/Industries Batteries
These kinds of batteries are used to power battery-powered vehicles or to power high-power devices in industries. These kinds of batteries have high energy density in the range of 100120 Wh/kg.
Batteries like lead-acid batteries, nickel-iron batteries silver-zinc batteries, fall into this kind, and also various high-temperature batteries are under development which also comes under this kind of batteries.
Stationary Batteries
Stationary batteries are used in stationary systems, such as standby power systems and load-leveling systems. These kinds of batteries are used to power when the load is low or extra power is available and provide extra power to the system when the load is very high or no power is available.
Applications of Batteries
The range and diversity of batteries used as a source of electrical power is truly enormous, reflecting the immense range of uses of electrical power. The diverse range of applications has spawned a multitude of specialist batteries, each optimized for a particular use.
Some of the applications for which unique batteries have been are mentioned below.
- Miniature Batteries: These batteries have energy of 100 mWh- 2 Wh and are used in applications like electric watches, calculators, and implanted medical devices.
- Batteries for portable equipment: These batteries have the energy of 2Wh- 100 Wh and are used in applications like flashlights, toys, power tools, portable radios and TVs, mobile phones, camcorders, laptops, and cordless devices.
- SLI Batteries: These batteries have the energy of 100 Wh- 600 Wh and are used in applications like Cars, trucks, buses, lawn movers, and wheelchairs.
- Vehicle Traction Batteries: These batteries have the energy of 20Wh- 630 kWh and are used in applications like EVs, HEVs, PHEVs, forklifts, and locomotives.
- Stationary Batteries: These batteries have energy of 250 Wh- 5 MWh and are used in applications like emergency power, local energy storage, remote relay stations, communication base stations, and UPS.
- Special Purpose Batteries: These Batteries are used in submarines, and satellites and they have an energy range of 3 MWh.