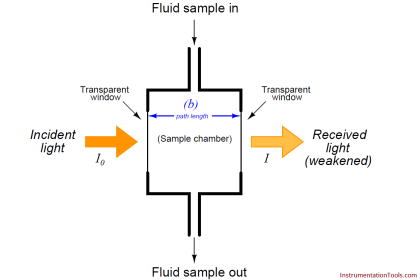
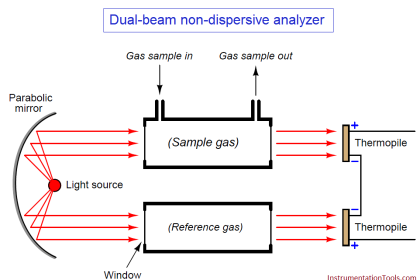
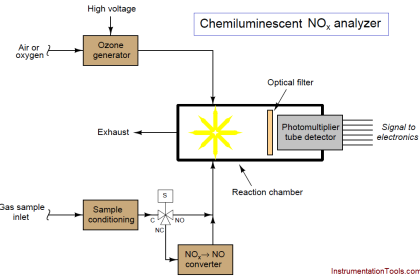
The sensor has three electrodes: a measuring electrode (gold), a counter electrode (gold), and a reference electrode (Ag/AgCl). A precise potential is built up between the measuring and the reference electrode. The measuring electrode starts polarising, i.e. ions collect close to the electrode to neutralize the electrical field. After polarisation the electrical current decreases to 0 mA as long as the polarising layer is not changed.
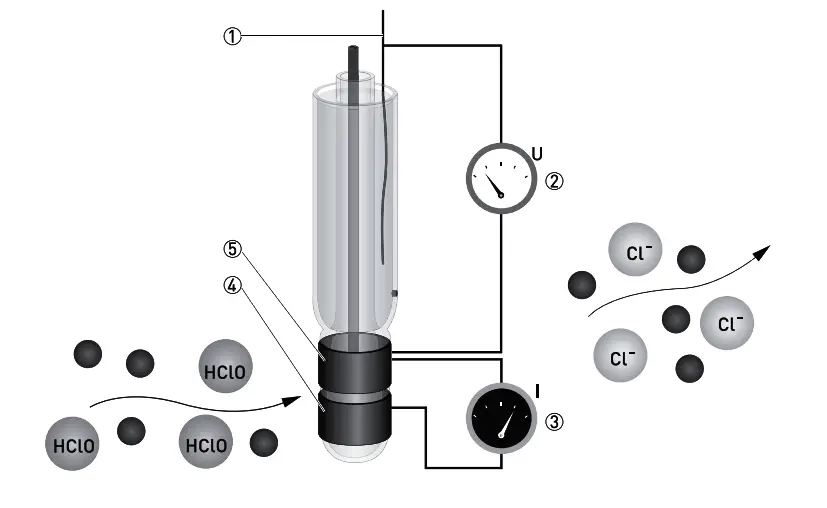
Image Credits : krohne
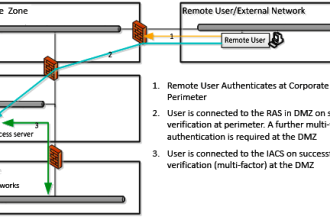
Parts :
- Reference electrode
- Applied chlorine specific potential
- Current needed to maintain the constant potential
- Counter electrode
- Measuring electrode
Free chlorine molecules that hit the surface of the measuring electrode take a defined portion of the charge with them, changing the measuring potential. The signal converter constantly measures the potential between measuring and reference electrode and immediately readjusts the potential as soon as it begins to change. The current needed to maintain a constant potential is directly correlated to the free chlorine concentration in the measuring medium.
Free chlorine (chlorine dissolved in water) changes its chemical composition depending on the pH value of the water. The pH value has consequences for the disinfection strength: with increasing pH the disinfection strength decreases.
- below pH3: Chlorine gas (Cl2)
- between pH3 and pH8: Hypochlorous acid (HClO)
- above pH8: Hypochlorite (ClO- )
In order to obtain a reliable free chlorine measurement you should either control or compensate the pH value of the measuring medium. Because the pH measurement is temperature dependent, it also makes sense to measure the temperature.
Source : krohne