We all know that batteries are used in automobiles. But, if you research properly, then you will find out that one of the most generally used types of batteries in an automobile is a lead acid battery. This chemical solution is so immensely popular due to the properties that it finds its use in almost every automobile battery.
In this post, we will learn the concept of lead-acid batteries.
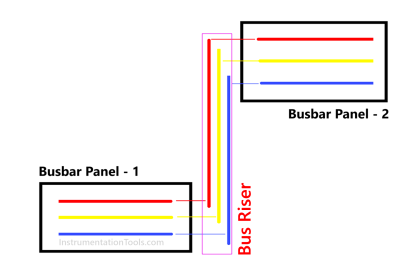
Lead Acid Battery
A battery is a device that converts some chemical energy into electrical energy. So, the chemical used will require some good conduction properties to produce electricity.
As the name implies, lead acid is a chemical that uses a combination of lead and sulphuric acid to generate electricity. This battery is rechargeable. You must have seen its structure, but it is mostly confusing due to its complex look.
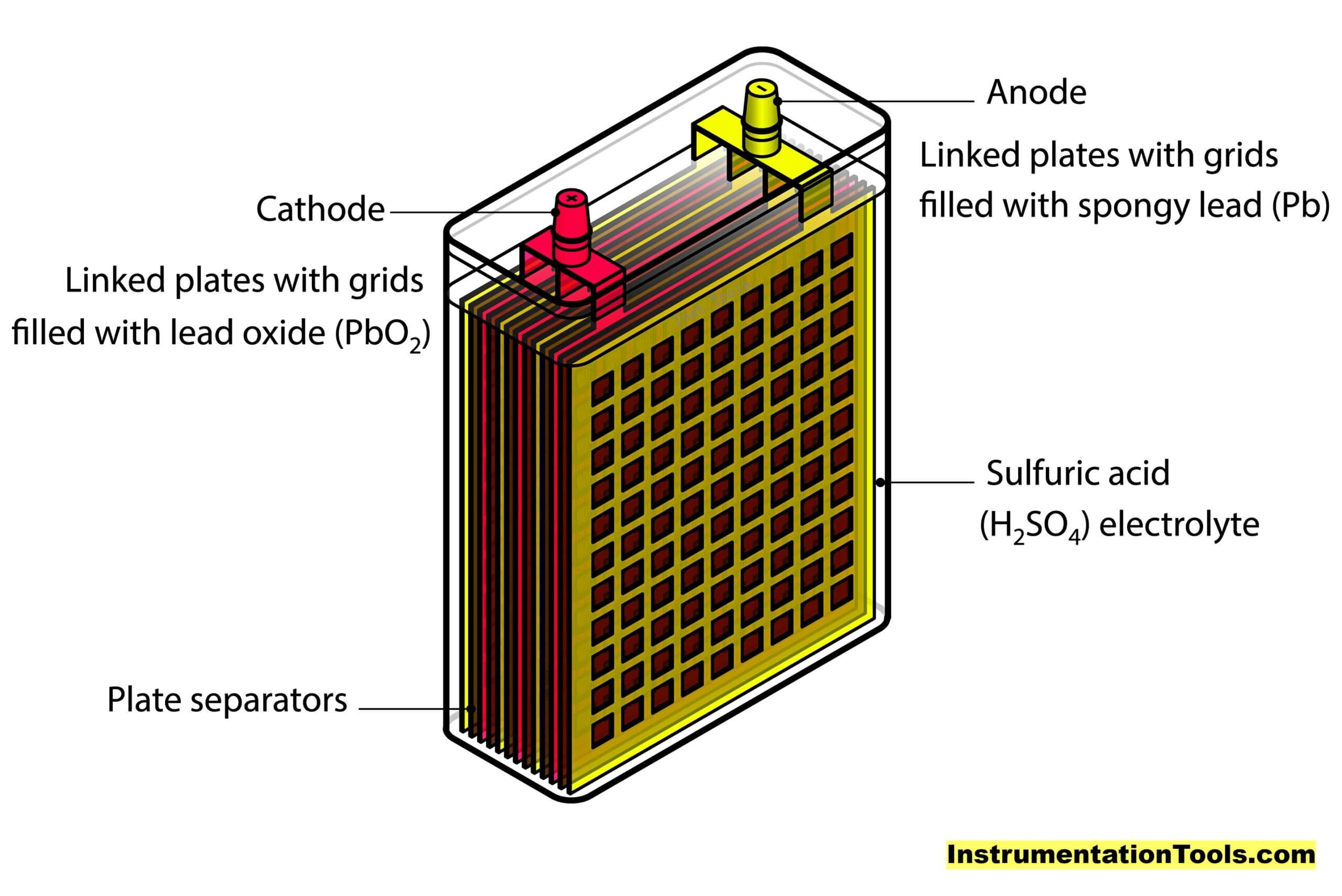
Let us simplify it in a better way. It can just be thought of as a vessel containing sulphuric acid, in which the lead plates (oppositely charged) are submerged.
Due to this submergence, a chemical reaction is produced which generates electricity. We will look into this in detail further.
These batteries have some demerits like – only a few years lifespan, being highly inflammable, less environment-friendly, great care in disposing of acids, and slow charging.
But, it is still one of the most widely used batteries due to the corresponding advantages they offer.
Let us understand them further below.
How does a Lead Acid Battery Work?
First of all, let us get through the basic concept of chemical reactions in electricity. As you know, an atom comprises protons, neutrons, and electrons. Protons are positively charged, neutrons have no charge, and electrons are negatively charged.
In our case, we will see the atom of lead metal. When this lead metal is submerged in a chemical, its atoms will either merge or break. But, how can we determine whether first of all the metal was positively charged or negatively charged?
Well, it depends on the ratio of protons and electrons. If the number of protons is more than electrons, then the atom is positively charged; and if the number of electrons is more than protons, then the atom is negatively charged.
Components of a Lead Acid Battery
Coming back to our topic, the main four components of a lead acid battery are –
- A negative electrode plate (spongy/porous lead)
- A positive electrode plate (lead oxide),
- An electrolyte solution of sulphuric acid and water, and
- A separator (for separating both plates).
The chemical liquid is used to trigger atomic charge activities with respect to lead metal. The positively charged plate (cathode) has atoms of lead and oxygen (lead oxide); whereas the negatively charged plate (anode) has atoms of only lead.
Sulphuric acid has the atoms of water (hydrogen–oxygen) and sulphate (hydrogen – sulphur). Refer to the below image for more understanding.
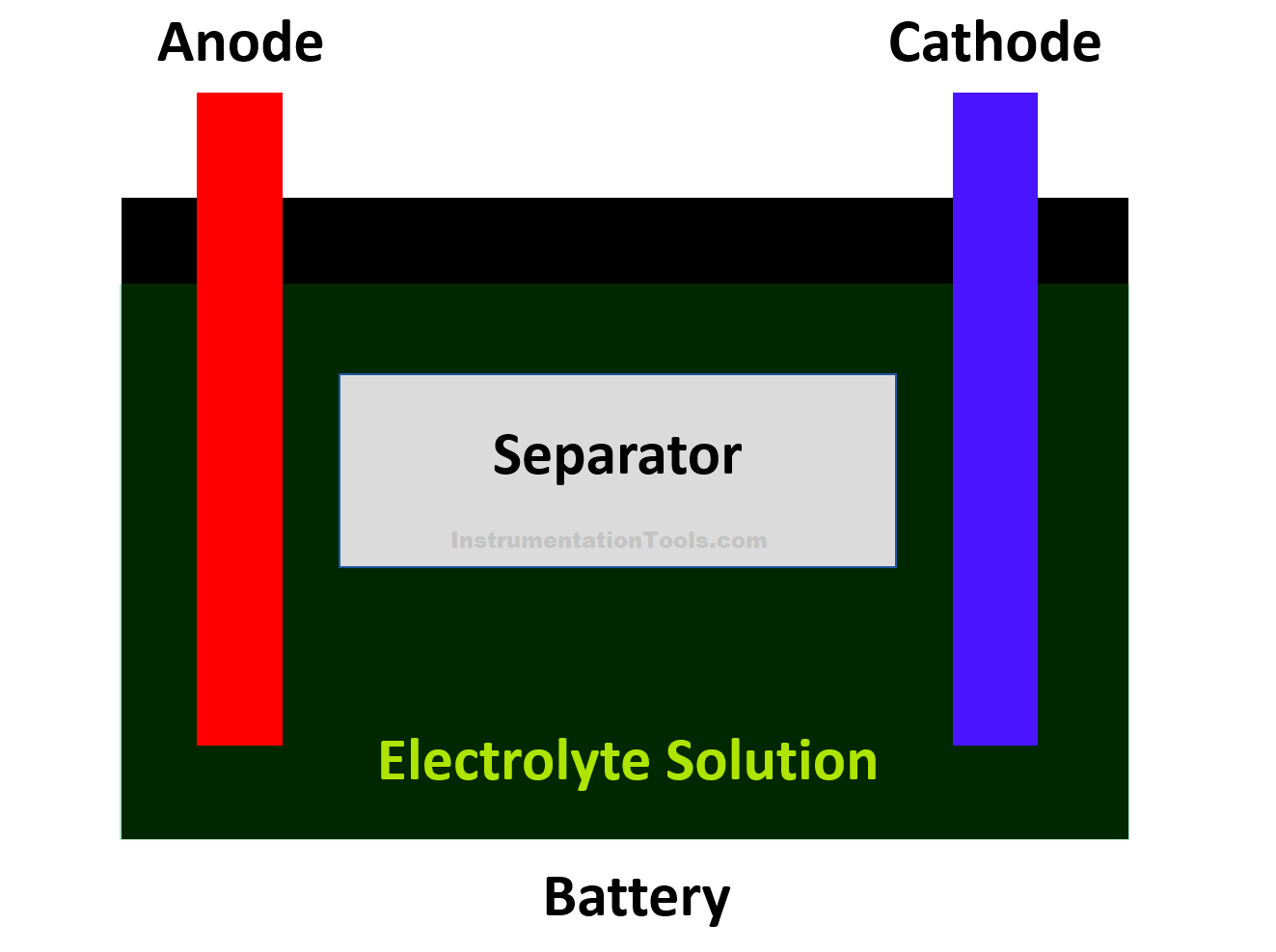
Now, let us start from the cathode point. The sulphate atom from the acid will react with a lead atom in the cathode to form lead sulphate in the plate. In exchange, the oxygen atoms from the cathode will react with the hydrogen atom in the acid to form water in the acid.
Now, let us move to the anode point. The sulphate atom from the acid will react with the lead atom in the anode to form lead sulphate in the plate. But, as you know, there was only a lead atom in the anode. So, some electrons from the lead sulphate will move to the upper layer of the negatively charged anode.
As the exchange is going on on the cathode side, due to no exchange on the anode side, more and more electrons will start to accumulate in the upper layer of the anode. This will start to create a potential difference between the anode and cathode.
When you then connect two terminal wires across the plates, then electricity will thus start to flow and the load connected in the automobile like a light, horn, or music player will get energized to function.
For additional information, let us also not forget the role of a separator. It just insulates the two plates but allows ion exchange in its layer through the sulphuric acid layer.
Now, imagine a situation where the electrons are just accumulating from the chemical acid and starting to increase in a vast amount. After some interval of time, the atoms will start to deplete in the chemical, making it diluted.
If there are no atoms for exchange, then there will be no further reaction; and thus, no further electricity will be generated. But, this complete action can simply be reversed with the help of an alternator.
Example
An alternator in an automobile is a device that converts the mechanical energy of a running engine into electrical energy and feeds back the supply to the battery to recharge it. Now, incoming electrons from the alternator will flow in the reverse direction from the cathode to the bottom layer of the anode.
The complete cycle of exchange discussed earlier will reverse and bring back all the atoms in their original positions in the same numbers. Then, the reaction process again starts to generate electricity and the cycle thus repeats continuously as long as the alternator is running.
Once the engine stops, then the battery will supply the remaining power in it into the loads with the engine off condition. Once the battery is discharged to its full capacity for fulfilling load demands (which means atoms diminished from the chemical), then electricity generation will stop. The battery will then be required to either recharge itself from an external power source or needs to be replaced it.
The lead cells come in the standard package of 6V, 12V, or 24V battery supply.
Lead Acid Battery Maintenance
Follow the following points related to the general maintenance of a lead-acid battery.
- Do not overcharge the battery. If it is overcharged, then after some period of time, it will start to be deprived of atoms in the chemical and then eventually, it will become unusable.
- The same opposite way, do not discharge it immediately. Constant charging and discharging of a battery can make it unusable after some period of time.
- Do not store the battery at a low charge, otherwise, a situation called sulfation will be created where crystals are formed inside the chemical which will hamper the battery capacity.
- Always keep the battery in a properly humid area, not too dry. Otherwise, as hydrogen gas is created inside the batteries, it is explosive in nature and if confined to a dry and small area, then there are high chances of batteries exploding.
- Apart from humidity, temperature also matters. Storing batteries in high-temperature areas will increase their internal discharge, eventually draining the batteries in non-usage too. Reversely, storing it in cold temperatures will freeze it and eventually, make it non-usable. So, always store them at proper room temperature.
- When gas bubbles start to appear on the plates of what are called flooded lead acid batteries, this indicates the battery is fully charged and it starts to split the water molecules into hydrogen (negative plate) and oxygen (positive plate).
Advantages of Lead-Acid Battery
Some of the advantages of a lead-acid battery are mentioned below.
- Lead and sulphuric acid are the simplest and cheapest chemicals to work with. So, bulk batteries are always made with this chemical solution. They provide the lowest cost per unit capacity for rechargeable cells.
- They have a very large current capacity, which is ideal for starting engines.
- It is more prone to physical stress and overcharging.
- It comes in a wide variety of sizes and specifications.
In this way, we understand how a lead acid battery works.
If you liked this article, then please subscribe to our YouTube Channel for Electrical, Electronics, Instrumentation, PLC, and SCADA video tutorials.
You can also follow us on Facebook and Twitter to receive daily updates.
Read Next:
- Basics of Electrical Transformer
- Routine Tests of Transformer
- Importance of Neutral Wire
- Power and Instrument Cables
- What Happens Pump Runs Dry?