Since process chromatographs have the ability to independently analyze the quantities of multiple species within a chemical sample, these instruments are inherently multi-variable.
A single analog output signal (e.g. 4-20 mA) would only be able to transmit information about the concentration of any one species (i.e. any one peak) in the chromatogram.
This is perfectly adequate if only one species concentration is of interest in the process, but some form of multi-channel digital (or multiple analog outputs) transmission is necessary to make full use of a chromatograph’s ability.
Legacy chromatographs have multiple 4-20 mA analog output channels (one for each compound), while most modern chromatographs provide the option of digital bus communication (e.g. Modbus, FOUNDATION Fieldbus, etc.) to transmit data on multiple species concentrations to indicators, recorders, and/or controllers.
All modern chromatographs are “smart” instruments, containing one or more digital computers executing the calculations necessary to derive precise measurements from chromatogram data.
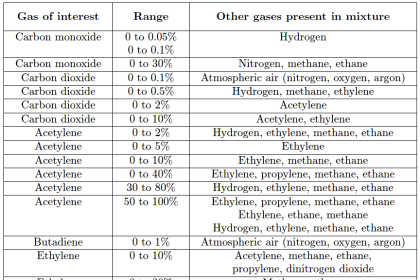
The computational power of modern chromatographs may be used to further analyze the process sample, beyond simple determinations of concentration or quantity. Examples of more abstract analyses include approximate octane value of gasoline (based on the relative concentrations of several species), or the heating value of natural gas (based on the relative concentrations of methane, ethane, propane, butane, carbon dioxide, helium, etc. in a sample of natural gas).
A very common industrial application of chromatographs is the monitoring and control of separation processes such as distillation (or “fractionation”) columns. The purpose of any separation process is to take a mixture or a solution and force some of its constituent compounds apart into different fluid streams.
The ability of a chromatograph to measure multiple species within a sample makes it ideally suited for the task of quantifying the purity of the separated species exiting the separation process.
For example, a chromatograph may be used to analyze the purity of alcohol output by the fractionation column in a distillery, quantifying alcohol concentration, water concentration, and even the concentrations of various aromatic and flavoring compounds within the distillate fluid.
That data may be then used to alter some of the controlled parameters of the fractionation process such as feed flow rate, pressure, temperature gradient, etc. to achieve the desired product composition.
Another industrial application of chromatography is the monitoring and control of chemical reaction processes. Once again we have a single instrument able to measure the concentration of desired product exiting the reaction, as well as unprocessed reactant species and also undesired products in the same product stream. This data may then be used to control parameters in the chemical reactor to optimize the reaction taking place there.
It should be noted that although gas chromatography (GC) is far more prevalent in online industrial process analysis than liquid chromatography (LC), this does not mean the GC technique is limited to the analysis of process fluids existing in the gas phase alone.
Gas chromatographs are often used to analyze the composition of liquid process samples, by first boiling that liquid sample within the analyzer so it may be analyzed in gaseous form.
This means many of the species within the GC must be operated at temperatures exceeding the boiling point of the lowest-boiling-point substance in the sample. While this poses certain technical challenges, it is nevertheless common practice in many industries.
The following photograph shows a gas chromatograph (GC) used to determine the heating value of natural gas at a natural gas pipeline compression facility. The entire instrument, from floor level to the top of the black box enclosing the chromatograph’s column, is about 6 feet in height:
This particular GC is used by a natural gas distribution company as part of its pricing system. The heating value of the natural gas is used as data to calculate the selling price of the natural gas (dollars per standard cubic foot), so the customers pay only for the actual benefit of the gas (i.e. its ability to function as a fuel) and not just volumetric or mass quantity.
No chromatograph can directly measure the heating value of natural gas, but the analytical process of chromatography can determine the relative concentrations of compounds within the natural gas. A computer, taking those concentration measurements and multiplying each one by the respective heating value of each compound, derives the gross heating value of the natural gas.
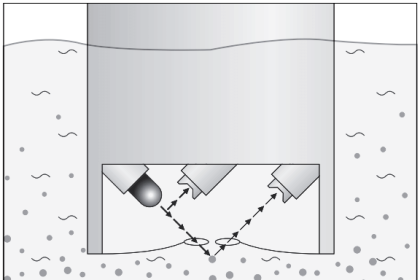
Although the column cannot be seen in this photograph of the GC, several high-pressure steel “bottles” may be seen in the background holding carrier gas used to wash the natural gas sample through the column.
A typical gas chromatograph column appears in the next photograph. It is nothing more than a stainless-steel tube packed with an inert, porous filling material:
This particular GC column is 28 feet long, with an outside diameter of only 1/8 inch (the tube’s inside diameter is even less than that). Column geometry and packing material vary greatly with application.
The many choices intrinsic to column design are best left to specialists in the field of chromatography, not the average technician or even the average process engineer.