In a lead-acid battery, two types of lead are acted upon electro-chemically by an electrolytic solution of diluted sulfuric acid (H2SO4). The positive plate consists of lead peroxide (PbO2), and the negative plate is sponge lead (Pb), shown in Figure 4.
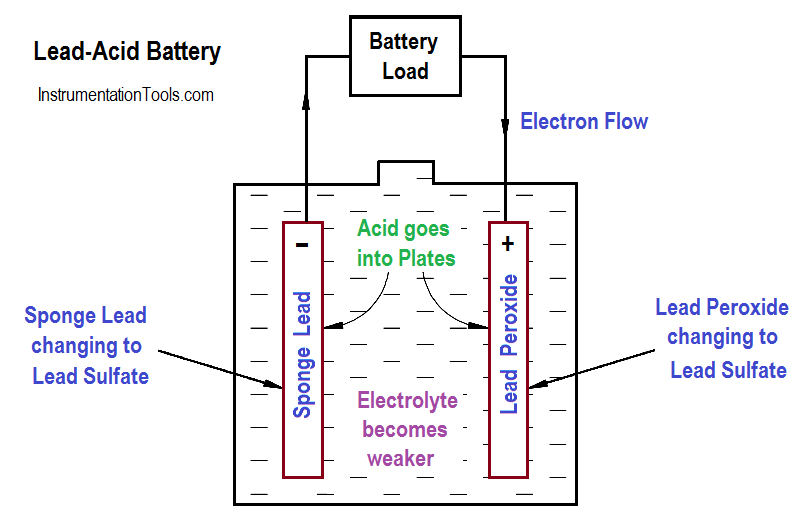
Figure 4 : Chemical Action During Discharge
When a lead-acid battery is discharged, the electrolyte divides into H2 and SO4 combine with some of the oxygen that is formed on the positive plate to produce water (H2O), and thereby reduces the amount of acid in the electrolyte. The sulfate (SO4) combines with the lead (Pb) of both plates, forming lead sulphate (PbSO4), as shown in Equation.
As a lead-acid battery is charged in the reverse direction, the action described in the discharge is reversed. The lead sulphate (PbSO4) is driven out and back into the electrolyte (H2SO4). The return of acid to the electrolyte will reduce the sulphate in the plates and increase the specific gravity. This will continue to happen until all of the acid is driven from the plates and back into the electrolyte, as shown in below Equation and Figure 5.
Figure 5 : Chemical Action During Charging
As a lead-acid battery charge nears completion, hydrogen (H2) gas is liberated at the negative plate, and oxygen (O2) gas is liberated at the positive plate. This action occurs since the charging current is usually greater than the current necessary to reduce the remaining amount of lead sulfate on the plates. The excess current ionizes the water (H2O) in the electrolyte. Since hydrogen is highly explosive, it is necessary to provide adequate ventilation to the battery whenever charging is in progress. Also, no smoking, electric sparks, or open flames are allowed near a charging battery.
The decrease in specific gravity on discharge is proportional to the ampere-hours discharged. While charging a lead-acid battery, the rise in specific gravity is not uniform, or proportional, to the amount of ampere-hours charged (Figure 6).
Figure 6 : Voltage and Specific Gravity During Charge and Discharge
The electrolyte in a lead-acid battery plays a direct role in the chemical reaction. The specific gravity decreases as the battery discharges and increases to its normal, original value as it is charged. Since specific gravity of a lead-acid battery decreases proportionally during discharge, the value of specific gravity at any given time is an approximate indication of the battery’s state of charge.
To determine the state of charge, compare the specific gravity, as read using a hydrometer, with the full charge value and the manufacturer’s published specific gravity drop, which is the decrease from full to nominal charge value.
Example:
A lead-acid battery reads 1.175 specific gravity. Its average full charge specific gravity is 1.260 and has a normal gravity drop of 120 points (or.120) at an 8 hour discharge rate.
Solution:
Fully charged – 1.260
Present charge – 1.175
The battery is 85 points below its fully charged state. It is therefore about 85/120, or 71%, discharged.
Learn an example PLC program to control a pump based on level sensors using ladder…
In the PLC timer application for security camera recording, when motion is detected then camera…
In this example, we will learn batch mixing with PLC ladder logic program using timer…
This PLC example on manufacturing line assembly is an intermediate-level PLC program prepared for the…
In this article, you will learn the PLC programming example with pushbutton and motor control…
This article teaches how to convert Boolean logic to PLC programming ladder logic with the…