Detection System of Gas Chromatography Questions and Answers
1. Which of the following is not an ideal characteristic of a detector used in gas chromatography?
a) Linear response to the solutes
b) Short response time
c) High reliability
d) Sensitive to the changes in the flow rate of carrier gas
Answer: d
Explanation: The detector used in gas chromatography must be insensitive to the changes in flow rate of carrier gas. There are many detectors used in gas chromatography.
2. Which of the following is not a type of detector used in gas chromatography?
a) Argon ionisation detector
b) Thermal conductivity detector
c) UV visible spectrometric detector
d) Electron capture detector
Answer: c
Explanation: UV visible spectrometric detector is not used in gas chromatography. It is used in liquid chromatography.
3. Which of the following detectors have high sensitivity to all organic compounds?
a) Sulphur chemiluminescense detector
b) Thermionic emission detector
c) Flame ionization detector
d) Argon ionisation detector
Answer: c
Explanation: Flame ionization detector has high sensitivity to all organic compounds. It is the commonly used detector for gas chromatography.
4. Which of the following is not the advantage of thermal conductivity detector used in gas chromatography?
a) Simple in construction
b) High sensitivity
c) Large linear dynamic range
d) Non-destructive character
Answer: b
Explanation: Thermal conductivity detector has relatively low density when compared to other detectors used in gas chromatography. It is based on the principle that all gases conduct heat in varying degrees.
5. Which of the following detectors is widely used to detect environmental samples like chlorinated pesticides and polychlorinated biphenyls?
a) Flame ionization detector
b) Thermal conductivity detector
c) Argon ionisation detector
d) Electron capture detector
Answer: d
Explanation: Electron capture detector is used to detect environmental samples like polychlorinated biphenyls and chlorinated pesticides. It is highly sensitive to molecules containing functional groups such as halogen and phosphorous.
6. In which of the following detector is the eluent mixed with hydrogen and burned and then mixed with ozone and its intensity is measured?
a) Sulphur chemiluminescense detector
b) Thermal conductivity detector
c) Flame ionization detector
d) Electron capture detector
Answer: a
Explanation: In Sulphur chemiluminescense detector, the eluent is mixed with hydrogen and burned and then mixed with ozone and its intensity is measured. The resultant is a measure of sulphur compounds present.
7. Filter photometer detector is primarily responsive to which of the following compounds/elements?
a) Volatile sulphur or phosphorous compounds
b) Nitrogen
c) Halogen
d) Potassium
Answer: a
Explanation: Flame photometric detector is primarily responsive to volatile sulphur or phosphorous compounds. It is also responsive to tin and nitrogen.
8. Which of the following detector uses ultraviolet radiation from lamps to produce ionisation of solute molecules?
a) Sulphur chemiluminescense detector
b) Thermal conductivity detector
c) Photo ionization detector
d) Electron capture detector
Answer: c
Explanation: Photo ionization detector uses ultraviolet radiation from lamps to produce ionisation of solute molecules. The current produced is measured and recorded.
9. Flame ionisation detector is also known as Katharometer.
a) True
b) False
Answer: b
Explanation: Thermal conductivity detector is known as Katharometer. It uses heated filament as sensing element and it is placed in the emerging gas stream.
10. Thermionic emission detector used in gas chromatography is most sensitive to which of the following elements?
a) Nitrogen
b) Phosphorous
c) Halogen
d) Carbon
Answer: b
Explanation: Thermionic emission detector used in gas chromatography is most sensitive to phosphorous. It is 500 times more sensitive to phosphorous than Flame ionization detector.
11. Which of the following detectors has a non-volatile bead of rubidium silicate placed above the flame tip?
a) Argon ionisation detector
b) Thermionic emission detector
c) Flame ionization detector
d) Electron capture detector
Answer: b
Explanation: Thermionic emission detector has a non-volatile bead of rubidium silicate placed above the flame tip. It is maintained at about 180V with respect to the collector.
12. In which of the following detectors, the carrier gas is excited by a radioactive source and the atoms of carrier gas are excited to metastable state?
a) Argon ionisation detector
b) Thermionic emission detector
c) Flame ionization detector
d) Electron capture detector
Answer: a
Explanation: In Argon ionisation detector, the carrier gas is excited by a radioactive source and the atoms of carrier gas are excited to metastable state. It uses argon as carrier gas.
13. Which of the following is not used as a heating element in Thermal conductivity detector?
a) Platinum
b) Gold
c) Graphite
d) Tungsten wire
Answer: c
Explanation: Graphite is not used as heating element in Thermal conductivity detector. Platinum, gold and tungsten wire are used as heating elements.
14. Electron capture detector is much less susceptible to contamination when nickel is used instead of tritium.
a) True
b) False
Answer: a
Explanation: Electron capture detector is much less susceptible to contamination when nickel is used instead of tritium. The sensitivity of nickel is less than that of tritium.
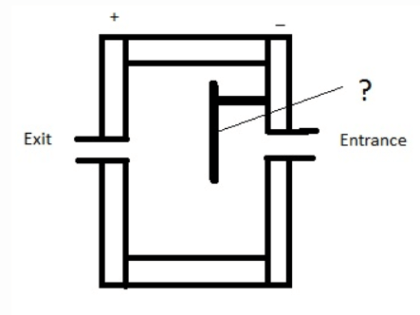
15. Given below is a diagram of electron capture detector. Identify the unmarked component in the diagram.
a) Glass shield
b) Electrode
c) Quartz shield
d) Radioactive β- emitter
Answer: d
Explanation: The unmarked component is Radioactive β- emitter. Nitrogen and hydrogen are the best carrier gases for these detectors.