There are different methods you can use to form protective coatings over objects. These include physical vapor deposition (PVD) and chemical vapor deposition (CVD). Of these methods, CVD is a popular and versatile technique to deposit good-quality covering.
In this guide, we’ll give you deeper insights into how chemical vapor deposition (CVD) works, its benefits, and its disadvantages. But first, let’s look at what CVD is.
What is Chemical Vapor Deposition?
Chemical vapor deposition (CVD) is a widely used method of creating high-quality coatings on the surfaces of objects (“substrates”) in a reaction chamber. This technique involves the use of the chemical reactions of one or more volatile precursors with heated substrates to deposit thin-film coatings on these substrates.
In chemistry, volatile precursors are substances in gaseous or vapor states. These precursors can be combined with inert gases like argon (Ar) or helium (He) to prevent unwanted surface reactions (e.g., oxidation) from degrading precursors. Thus, these inert gases help carry volatile precursors safely to the chamber.
For example, an aluminum powder supplier can synthesize aluminum oxide (Al2O3) from aluminum trichloride (AlCl3), oxygen (O2), and argon. But argon doesn’t react with AlCl3 and O2 to create chemical compounds. Instead, the CVD process uses this inert gas to dilute and transport oxygen to the reactor.
There are many types of CVD processes, like plasma-assisted CVD and atmospheric pressure CVD. Although these processes differ in operating conditions, they often require three main factors to deposit bulk materials successfully:
- Volatile precursors: Precursors in CVD processes must be volatile. This is because CVD will use gas molecules to deposit solid coatings. This technique is different from physical vapor deposition (PVD), which bombards solid source materials into atoms and deposits these atoms on substrates.
- Vacuum chamber: A vacuum environment has low pressure, limiting unwanted reactions and creating a more uniform thickness of deposited materials on substrates.
- Elevated temperature: Elevated temperature is needed in CVD processes because precursors deposit at very high temperatures, i.e., silane (SiH4) at 300-500oC or TEOS (Si(OC2H5)4) at 650-750oC. Further, high temperatures can enhance the reaction rate. Under high-temperature conditions, gas molecules will move faster and collide with each other more often. The rate of reaction will increase accordingly.
CVD is commonly used to produce coatings for objects as these coatings have high quality and low porosity levels. With these features, CVD coatings have a wide variety of applications in electronics and other industries.
For example, CVD coatings help protect objects (e.g., electronic components) against water, high temperature, and corrosion. Further, the semiconductor sector often employs CVD to create high-performant thin films and conductive parts (e.g., contacts or plugins). In the jewelry industry, CVD can be applied to synthesize diamonds by depositing the carbon atoms of a precursor gas on substrates.
How Does CVD Work?
The CVD coating process consists of the following fundamental steps:
1. A substrate that needs coating will be placed inside a reaction chamber. Then, a producer will add a mixture of volatile precursors and inert gases to the reaction chamber.
2. Then, the substrate is heated by resistive heating (e.g., tube furnaces), microwave power, lasers, or plasma. At the same time, the pressure inside the chamber will be reduced to activate the chemical reactions of the gas mixture.
3. After that, the gas mixture will decompose or react with the substrate material to deposit thin-film coatings.
4. The chemical reactions of the gas mixture can produce volatile byproducts. For example, after the CVD process deposits tungsten (W) from tungsten hexafluoride (WF6) and hydrogen (H2), hydrogen fluoride (HF) is created as a gaseous byproduct as follows:
WF6 + 3 H2 → W + 6 HF
The gaseous byproduct is then removed from the vacuum chamber and processed properly to avoid polluting the environment.
Chemical Coating Pros and Cons
Chemical vapor deposition comes with potential benefits and unwanted drawbacks. Let’s discuss them in the next part of this guide:
Pros of Chemical Coating
CVD allows a mil-spec supplier to build coatings with uniform thickness even over-complicated shapes. Thanks to CVD, the supplier can apply thin-film coatings to the insides, undersides, high-aspect-ratio holes, and other complex features of materials.
This is something PVD cannot do.
This is because PVD is a line-of-sight coating process. This means that the particles that are used to form coatings move directionally from solid source materials to substrates. The result is that PVD cannot create uniform thickness on all faces of substrates.
To make substrates evenly coated with PVD, the manufacturer must rotate the substrates to ensure that all the molecules can cover all surfaces. This can ultimately be a waste of time and money.
To better understand how molecules coat an object in CVD and PVD, take a look at the figure below:
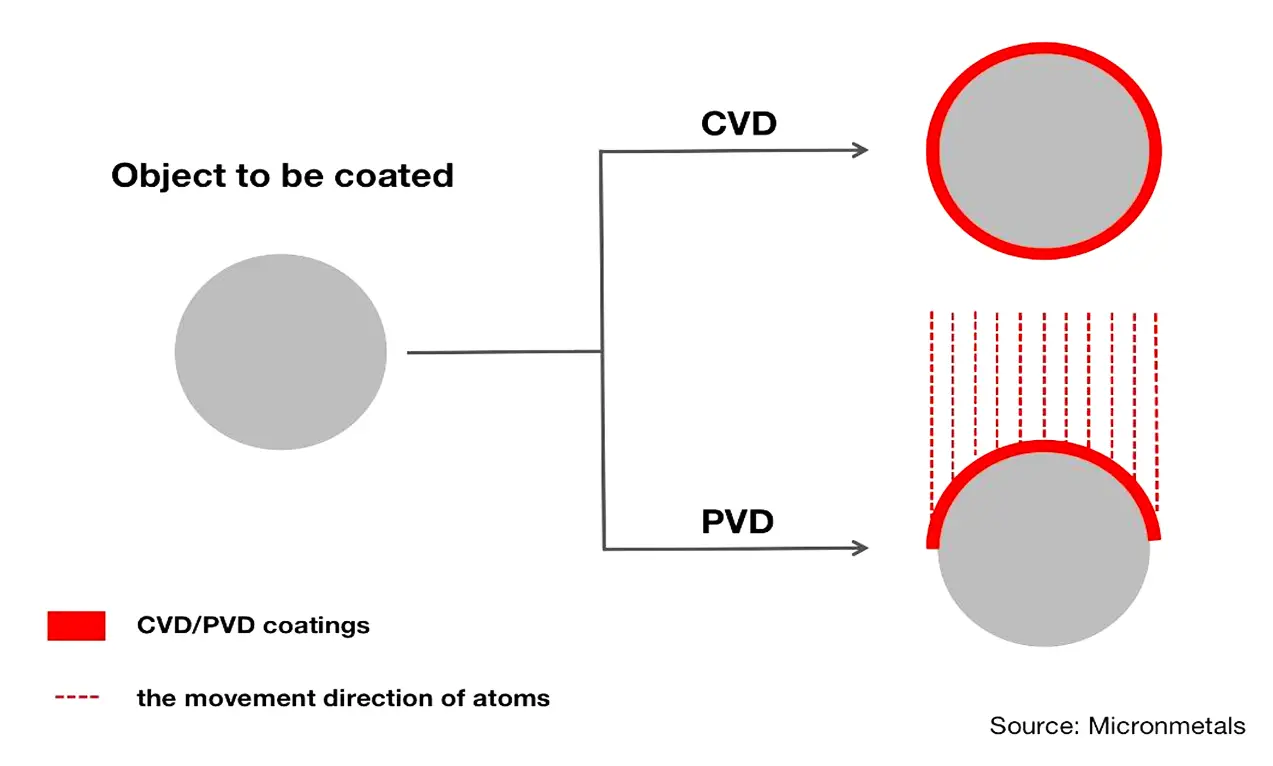
Another benefit of CVD is that CVD coatings have very high purity. This is because CVD processes involve the application of distillation techniques to evacuate impurities from gaseous precursors.
CVD coatings are also high-quality, waterproof, and fine-grained. Further, they are harder than similar materials produced by traditional manufacturing processes. This is because the reaction of volatile precursors with a substrate can create a stronger bond on the substrate’s surface.
Last but not least, CVD has high deposition rates. But it’s essential to modify the temperature and duration of CVD to control the coating’s thickness on substrates.
Cons of Chemical Coating
CVD also comes with some drawbacks.
Firstly, precursors must be volatile to decompose or react on substrates. But if these precursors are too volatile, they can evaporate before being delivered to the vacuum chamber. That’s why it’s vital to choose and preserve precursors to limit their evaporation.
Second, some CVD precursors like Cu(acac)2, B2H6, or Ni(CO)4 are poisonous, corrosive, and explosive. If these gaseous precursors are not preserved and are not delivered with caution, they can have a detrimental impact on the environment and the health of those exposed to these gases.
Third, gaseous byproducts such as HF, H2, or CO are very toxic. So, it’s imperative to process these gases properly when they’re released from the vacuum chamber.
Finally, CVD processes deposit thin-film coatings at very high temperatures. But some substrate materials have poor thermal stability. So, choosing substrate materials that cannot withstand high temperatures can make CVD processes fail.
For example, at 150oC, aluminum alloys start becoming weak. Meanwhile, some precursors like silane deposit in a temperature range of 300-500oC. If a manufacturer chooses silane as a precursor gas that reacts with the aluminum substrate, the chemical reaction can corrode the substrate. Thus, when choosing volatile precursors, the manufacturer must consider substrate materials and their thermal stability.
In Closing
Chemical vapor deposition is one of the most effective methods for creating protective coatings for electronic components and semiconductive parts.
A CVD is straightforward. But to make this coating process successful, it’s essential to strictly abide by guidelines about temperature, precursor volume, substrate materials, chamber material, pressure, and duration.
CVD has a wide variety of benefits, typically the ability to build uniform thickness over substrates. But its downside is some gaseous precursors and byproducts in CVD are toxic. So, those working with CVD processes must conform to a standard operating procedure (SOP) to ensure the safety of their health and the environment.