The pharmaceutical industry sector is known for its standards and precision tools and equipment that can match its exact requirements. In this industry, the quality and consistency of products are not just about maintaining business reputation, they are crucial for ensuring patient safety and efficacy of medications. This high-stakes field relies on a variety of sophisticated tools to meet its complex needs, from research and development to production and quality control.
The Role of Precision Tools
The main roles of precision instruments and tools in the pharmaceutical industry are listed below.
- Precision tools ensure accurate drug formulation.
- Vital for maintaining consistent quality in pharmaceuticals.
- Crucial in dosage measurement and control.
- Enhances efficiency in drug production.
- Key in adhering to regulatory standards.
- Important for ensuring patient safety.
- Integral in research and development of new drugs.
- Essential for sterile processing and packaging.
- Improves precision in chemical analysis.
- Key in automation of manufacturing processes.
- Aids in efficient quality control testing.
- Critical for drug stability testing.
- Supports accurate labeling and information dissemination.
- Facilitates compliance with international standards.
- Enables advanced drug delivery system development.
- Important for efficient and safe equipment cleaning.
- Crucial for environmental monitoring in production areas.
Pharmaceutical Industry
One key aspect of pharmaceutical manufacturing and quality assurance is the accurate measurement of various components and properties in drug formulations. This is where specialised tools come into play, offering the precision and reliability that this sector demands.
The Importance of Moisture Content Analysis
Moisture content in pharmaceutical products is a critical quality attribute, as it can significantly affect their shelf life, stability, and effectiveness. Excessive moisture can lead to the degradation of active pharmaceutical ingredients (APIs), while insufficient moisture may affect the binding properties in tablet formation. Therefore, controlling and measuring moisture content is essential in the pharmaceutical manufacturing process.
The process of moisture analysis must be accurate, reliable, and efficient. This is where Karl Fischer titration, a method developed by the chemist Karl Fischer, becomes invaluable. It’s a titration method specifically designed for precise moisture determination. Its accuracy and reliability make it a preferred choice in the pharmaceutical industry, where meeting strict regulatory standards is not just important but mandatory.
Karl Fischer Titrators
Metrohm is a leading provider of analytical instruments and services and offers a range of Karl Fischer titrators that are widely recognized for their precision and ease of use in moisture analysis. The Metrohm Karl Fischer titrators are designed to cater to the varying needs of pharmaceutical companies, from small-scale laboratories to large production facilities.
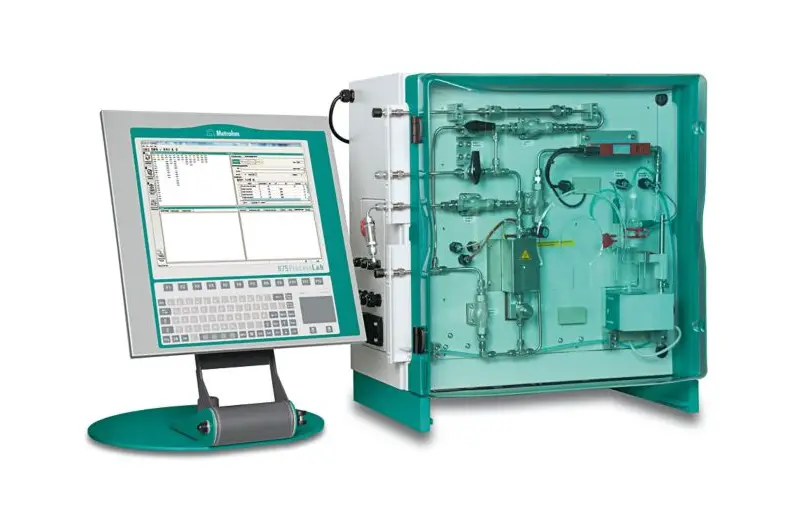
These titrators stand out for their accuracy in determining the moisture content of a wide range of pharmaceutical products. When dealing with powders, liquids, or solids, these instruments can deliver fast and reliable results. This accuracy is crucial in a field where the margin for error is minimal, and the implications of inaccuracy can be significant.
These titrators are known for their user-friendliness. They come with intuitive interfaces and software that simplify the process, making it easier for technicians to carry out analyses with minimal training. This user-centric design is particularly important in busy pharmaceutical laboratories where time and efficiency are of the essence.
Advanced Features and Compliance
Karl Fischer titrators are not just about performing moisture analysis; they are also about doing it in a way that aligns with the industry’s stringent compliance requirements. These instruments come equipped with features that support compliance with international standards and regulations, such as those set by the FDA and EMA.
One of the key aspects of compliance in the pharmaceutical industry is traceability and data integrity. Titrators are designed with these requirements in mind. They offer features like secure data logging, audit trails, and user management, ensuring that all data generated during the moisture analysis process is accurate, secure, and traceable.
These titrators are adaptable, with the capability to integrate seamlessly into existing laboratory systems and workflows. This flexibility makes them a practical choice for laboratories looking to upgrade their equipment without overhauling their entire system.
Advancements in Spectroscopy
Shifting focus from moisture analysis, another pivotal area in the pharmaceutical industry is the analysis of drug composition and quality. Spectroscopy involves the measurement of light as it interacts with matter and it plays a crucial role in this domain. This technology has evolved significantly, leading to more sophisticated and precise instruments that aid in the identification and quantification of substances in drug products.
Spectroscopy is particularly valuable in the pharmaceutical industry due to its non-destructive nature and its ability to provide detailed information about the chemical structure and composition of a sample. Advanced spectroscopic techniques such as nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, and infrared (IR) spectroscopy are widely used for drug testing and quality control.

NMR spectroscopy is instrumental in elucidating the molecular structure of pharmaceutical compounds. It allows scientists to identify the precise arrangement of atoms in a molecule, which is essential for understanding the drug’s pharmacological properties.
Mass spectrometry is highly effective in identifying and quantifying trace compounds in complex mixtures. This is particularly important in detecting impurities and degradation products that could compromise drug safety and efficacy.
Automation and Robotics in Pharmaceutical Manufacturing
Moving from analytical techniques to manufacturing processes, automation and robotics have revolutionized the pharmaceutical industry. These technologies have increased the efficiency, accuracy, and safety of drug production processes. In an industry where precision is important, the ability to automate repetitive and intricate tasks has been a game changer.
Robotics, for instance, is used for a range of applications in pharmaceutical manufacturing, including drug formulation, filling vials and syringes, packaging, and labelling. Automation in these processes not only enhances precision but also minimizes human error, thereby increasing the overall quality of the pharmaceutical products.
Automation has been instrumental in improving the scalability of production processes. As demand for pharmaceuticals grows globally, the ability to scale up production rapidly without compromising quality is crucial. Automated systems can be programmed to adjust to different production volumes, ensuring consistent product quality regardless of the scale.
Data Management and Digital Integration
In addition to physical tools and equipment, the pharmaceutical industry increasingly relies on digital technologies for data management and integration. With the growing complexity of pharmaceutical products and processes, managing vast amounts of data has become a critical task. Efficient data management systems are essential for storing, processing, and analyzing data generated during various stages of pharmaceutical development and manufacturing.
Digital integration plays a vital role in connecting various aspects of pharmaceutical operations. From research and development to manufacturing and distribution, integrating data across all stages ensures coherence and efficiency. This integration is particularly important for complying with regulatory requirements, as it allows for seamless tracking and documentation of the entire drug development and production process.
Emerging technologies like artificial intelligence (AI) and machine learning are also beginning to make their mark in the industry. These technologies are being used for drug discovery, predictive analysis in manufacturing processes, and even in personalizing medicine. AI algorithms can analyze large datasets to identify potential drug candidates or predict how different manufacturing variables can affect product quality.