Atomic Absorption Spectroscopy Questions & Answers
1. Which of the following is the principle of Atomic Absorption Spectroscopy?
a) Radiation is absorbed by non-excited atoms in vapour state and are excited to higher states
b) Medium absorbs radiation and transmitted radiation is measured
c) Colour is measured
d) Colour is simply observed
Answer: a
Explanation: Atoms in gaseous state absorb the radiation and are excited to higher state. Since, the higher state is unstable the atom returns the ground state with the emission of radiation which is measured.
2. In Atomic Absorption Spectroscopy, which of the following is the generally used radiation source?
a) Tungsten lamp
b) Xenon mercury arc lamp
c) Hydrogen or deuterium discharge lamp
d) Hollow cathode lamp
Answer: d
Explanation: Hollow cathode lamp is the source used in Atomic Absorption Spectroscopy. It emits stable and intense radiation.
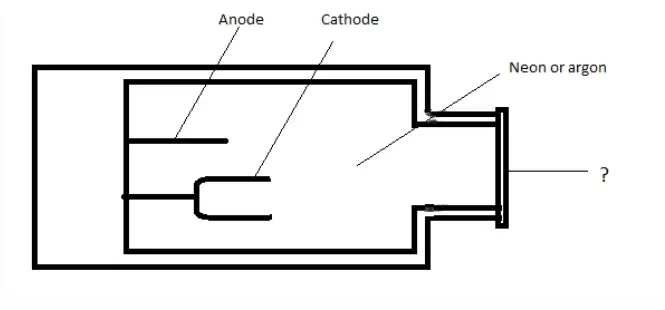
3. In Atomic Absorption Spectroscopy, with what material is the cathode in Hollow cathode lamp constructed?
a) Tungsten
b) Quartz
c) Element to be investigated
d) Aluminium
Answer: c
Explanation: The cathode in Hollow cathode lamp is constructed of the element to be investigated. The anode is made of tungsten.
4. How can the intensity of radiation be increased in Hollow cathode lamp?
a) Addition of non-conductive protective shield of mica
b) Addition of nitrogen to neon or argon in the lamp
c) Increasing the pressure of the filling gas
d) Changing the metal of the anode
Answer: a
Explanation: The intensity of radiation is increased in Hollow cathode lamp by the addition of non-conductive protective shield of mica. The protective shield can be made of glass too.
5. Which of the following is the function of the chopper in Atomic Absorption Spectroscopy?
a) To split the beam into two
b) To break the steady light into pulsating light
c) To filter unwanted components
d) To reduce the sample into atomic state
Answer: b
Explanation: The function of the chopper in Atomic Absorption Spectroscopy is to break the steady light into pulsating light. It is a rotating wheel placed between the flame and the source.
6. Which of the following is the function of Flame or Emission system in Atomic Absorption Spectroscopy?
a) To split the beam into two
b) To break the steady light into pulsating light
c) To filter unwanted components
d) To reduce the sample into atomic state
Answer: d
Explanation: The function of Flame or Emission system in Atomic Absorption Spectroscopy is to reduce the sample into atomic state. In Atomic Absorption Spectroscopy, the production of atomic vapour by flame is the most important phase.
7. Atomic absorption spectroscopy is also called as Absorption Flame Photometry.
a) True
b) False
Answer: a
Explanation: In Atomic Absorption Spectroscopy, sample is sprayed into the flame. Hence, it is called Absorption Flame Photometry.
8. Which of the following is not a component of emission system in Flame photometer?
a) Burner
b) Atomiser
c) Fuel gases and their regulation
d) Chopper
Answer: d
Explanation: Chopper is not a component of emission system in Flame photometer. The parts of flame photometer are burner, atomiser, fuel gases and their regulation and flame.
9. Which of the following is the function of atomiser in the emission system of Atomic Absorption Spectroscopy?
a) To split the beam into two
b) To break the steady light into pulsating light
c) To break large mass of liquid into small drops
d) To reduce the sample into atomic state
Answer: c
Explanation: The function of atomiser in the emission system of Atomic Absorption Spectroscopy is to break large mass of liquid into small drops. It also introduces liquid sample into the flame at a stable rate.
10. Which of the following is not a fuel used in flame photometry?
a) Acetylene
b) Propane
c) Hydrogen
d) Camphor oil
Answer: d
Explanation: The commonly used fuel gases in flame photometry are acetylene, propane and hydrogen. Oxygen supply is given to the fuel gases.
11. Which of the following is not the requirement of a good flame in flame photometer?
a) Liquid sample must be evaporated to form solid residue
b) Solid residue must decompose to form atoms
c) Atoms must be produced such that they have the ability to get excited to higher states
d) Atoms must be produced such that they are in stable state
Answer: d
Explanation: Atoms must be produced such that they have the ability to get excited to higher states. These atoms in higher states return to ground state with the emission of photons.
12. Atomic Absorption Spectroscopy is used for the analysis of metals.
a) True
b) False
Answer: a
Explanation: Atomic Absorption Spectroscopy is used for the analysis of metals.
13. Which of the following options explains the process of ‘sputtering’ that occurs in Hollow Cathode Lamp?
a) Positive ions collide with cathode surface and metal atoms from cathode are ejected
b) Negative ions collide with cathode surface and metal atoms from anode are ejected
c) Positive ions collide with negative ions and metal atoms from anode are ejected
d) Positive ions collide with negative ions and photons are ejected
Answer: a
Explanation: When potential is applied across the electrode, the gas filled in tube ionises and flow of current occurs. Positive ions collide with negatively charged cathode surface and metal atoms from cathode are ejected.
14. At what pressure should the gases in the sealed tube be maintained in the Hollow cathode lamp?
a) 1 to 5 torr
b) 20 to 30 torr
c) 40 to 50 torr
d) 50 to 55 torr
Answer: a
Explanation: It consists of a cylindrical cathode and an anode made of tungsten. The tube is sealed and neon and argon are filled at a pressure of 1 to 5 torr.
15. The diagram show below is the picture of Hollow cathode lamp. Identify the unmarked component.
a) Glass tube
b) Quartz window
c) Non- conducting glass
d) Mica shield
Answer: b
Explanation: The unmarked portion is Quartz window. The window can be made of quartz or borosilicate glass.











The section is very good. it helps the student to have a quick review of the topic.