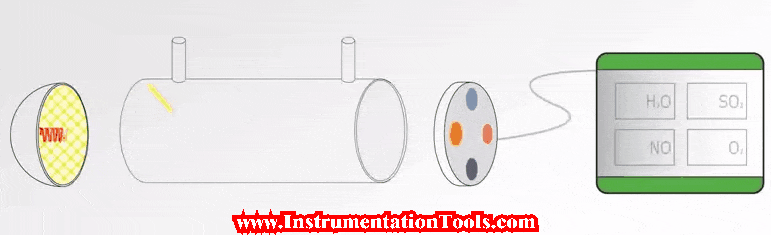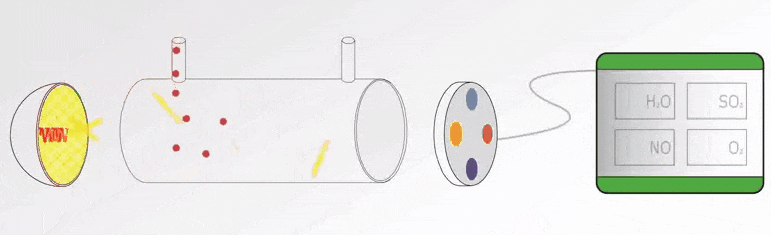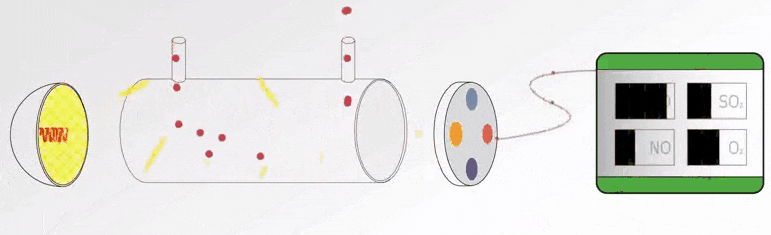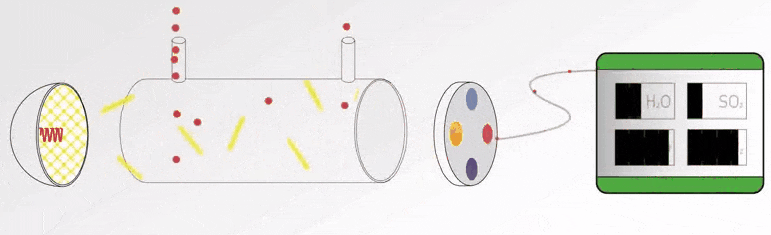
UV Visible Spectrometers Questions and Answers
1. Beer Lambert’s law gives the relation between which of the following?
a) Reflected radiation and concentration
b) Scattered radiation and concentration
c) Energy absorption and concentration
d) Energy absorption and reflected radiation
Answer: c
Explanation: Beer Lambert’s law gives the relation between Energy absorption and Concentration. It was proposed by Beer and Lambert.
2. In which of the following ways, absorption is related to transmittance?
a) Absorption is the logarithm of transmittance
b) Absorption is the reciprocal of transmittance
c) Absorption is the negative logarithm of transmittance
d) Absorption is a multiple of transmittance
Answer: c
Explanation: Transmittance is the ratio of the radiant power transmitted by a sample to the radiant power incident on the sample. Absorption is the negative logarithm of transmittance.
3. Which of the following is not a limitation of Beer Lambert’s law, which gives the relation between absorption, thickness, and concentration?
a) Concentration must be lower
b) Radiation must have higher bandwidth
c) Radiation source must be monochromatic
d) Does not consider factors other than thickness and concentration that affect absorbance
Answer: b
Explanation: The law is derived assuming that the radiation is monochromatic. So, if bandwidth increases it will create deviation.
4. Beer’s law states that the intensity of light decreases with respect to ___________
a) Concentration
b) Distance
c) Composition
d) Volume
Answer: a
Explanation: Beer’s law states that the intensity of light decreases with the concentration of the medium. It was stated by Beer.
5. Lambert’s law states that the intensity of light decreases with respect to __________
a) Concentration
b) Distance
c) Composition
d) Volume
Answer: b
Explanation: Lambert’s law states that the intensity of light decreases with respect to the concentration of the medium. It was stated by Lambert.
6. The representation of Beer Lambert’s law is given as A = abc. If ‘b’ represents distance, ‘c’ represents concentration and ‘A’ represents absorption, what does ‘a’ represent?
a) Intensity
b) Transmittance
c) Absorptivity
d) Admittance
Answer: c
Explanation: ‘a’ represents the absorption constant. It is also known as absorptivity.
7. Which of the following is not true about Absorption spectroscopy?
a) It involves transmission
b) Scattering is kept minimum
c) Reflection is kept maximum
d) Intensity of radiation leaving the substance is an indication of concentration
Answer: c
Explanation: In Absorption spectroscopy, reflection must also be kept minimum along with scattering. Amount of absorption depends on the number of molecules in the material.
8. Transmittance is given as T = P/Po. If Po is the power incident on the sample, what does P represent?
a) Radiant power transmitted by the sample
b) Radiant power absorbed by the sample
c) Sum of powers absorbed and scattered
d) Sum of powers transmitted and reflected
Answer: a
Explanation: P represents radiant power transmitted by the sample. Transmittance is the ratio of radiant power transmitted by the sample to the radiant power that is incident on it.
9. What is the unit of absorbance which can be derived from Beer Lambert’s law?
a) L mol-1 cm-1
b) L gm-1 cm-1
c) Cm
d) No unit
Answer: d
Explanation: Absorbance has no unit. The units of absorptivity, distance, and concentration cancel each other. Hence, absorption has no unit.
10. What is the unit of molar absorptivity or absorptivity which is used to determine absorbance A in Beer Lambert’s formula?
a) L mol-1 cm-1
b) L gm-1 cm-1
c) Cm
d) No unit
Answer: a
Explanation: The unit of absorptivity is L mol-1 cm-1. If the concentration is represented as gm per litre it becomes L gm-1 cm-1.